Abstract
Honey bees begin life working in the hive. At ≈3 weeks of age, they shift to visiting flowers to forage for pollen and nectar. Foraging is a complex task associated with enlargement of the mushroom bodies, a brain region important in insects for certain forms of learning and memory. We report here that foraging bees had a larger volume of mushroom body neuropil than did age-matched bees confined to the hive. This result indicates that direct experience of the world outside the hive causes mushroom body neuropil growth in bees. We also show that oral treatment of caged bees with pilocarpine, a muscarinic agonist, induced an increase in the volume of the neuropil similar to that seen after a week of foraging experience. Effects of pilocarpine were blocked by scopolamine, a muscarinic antagonist. Our results suggest that signaling in cholinergic pathways couples experience to structural brain plasticity.
Keywords: acetylcholine, Apis mellifera, foraging, mushroom body
Experience refines the structure of the brain. Well documented examples include the addition of higher order branches to the dendrites of cortical neurons in rats living in cages stocked with toys (1) and enlargement of the hippocampal formation subsequent to seed caching and retrieval in food storing birds (2). Numerous studies of insects have indicated that the ability of experience to shape the brain is not limited to relatively long-lived, large-brained vertebrates. The honey bee, Apis mellifera, is a case in point.
For the first 2 to 3 weeks of their roughly 6-week adult lives, worker bees perform activities within the hive such as brood care, food storage, and hive maintenance (3). They then become foragers and thereafter spend their days flying from the hive to gather nectar and pollen from flowers (4). Foraging requires assessment of the colony's nutritional needs, navigation by using the sun as a compass, recall of the location of the hive, acquisition of information about odors and colors of specific floral sources, and communication of the distance and direction of attractive food sources to nestmates via symbolic dances (5, 6).
There are many physiological changes that trigger or support the shift from the temperature- and humidity-regulated, dark, odordominated environment inside the hive to the ever-changing, sunlit world outside (7). Foraging is associated with growth of the neuropils of an insect brain region called the mushroom bodies (8–10). In bees with several weeks of foraging experience, the increase in neuropil volume is substantial, and may be equivalent to 50% of the volume of this structure in 1-day-old adult bees (10). As might be predicted, increased foraging experience is associated with improved foraging efficiency (11), although direct links between the structural changes in the brain and enhanced performance have yet to be made.
The mushroom bodies are located at the dorsal midline of the insect brain (12). They consist of densely packed clusters of interneurons, called Kenyon cells, and the neuropils defined by the axon terminals and dendritic arborizations of these neurons, the lobes and the calyces. Disruption of synaptic signaling in the mushroom bodies is associated with poor performance in certain tests of learning and memory, especially tasks involving the association of odors with punishment or reward (13–15). Study of the Drosophila mushroom bodies has led to the discovery of broadly conserved molecular mechanisms of learning and memory, including key functions for calcium/calmodulin-dependent adenylyl cyclase, protein kinase A, Notch, CREB, and the NMDA receptor (16, 17).
The fact that the increase in neuropil volume associated with foraging reflects increases in length and branching of Kenyon cell dendrites was demonstrated in a Golgi study that compared dendritic arborizations of Kenyon cells in age-matched foragers with different amounts of foraging experience (10). Neurogenesis is absent in the mushroom bodies of most adult insects, including the honey bee and, therefore, cannot account for any of the growth of these structures in foraging bees (18, 19).
The simplest model for experience-dependent growth of the mushroom bodies predicts that signaling through sensory pathways directly activated by foraging is the stimulus that triggers the growth of Kenyon cell dendrites. The mushroom bodies receive afferents from primary sensory regions of the brain, including the antennal lobes and the optic lobes (12, 20). The projections from the antennal lobe, which convey processed information from receptors on the antennae, are cholinergic (21). Expression of the gene encoding acetylcholinesterase by the Kenyon cells provides another indication of the importance of the cholinergic system to mushroom body functioning (22).
The neurotransmitter acetylcholine signals via distinct ionotropic (nicotinic) and metabotropic (muscarinic) receptors in both vertebrates and invertebrates, including insects (23, 24). In mammals, substantial evidence indicates a role for forebrain cholinergic mechanisms in learning and memory (25); in humans, loss of cholinergic function contributes to the loss of cognitive function seen in patients with Alzheimer's disease (26) and is a target for therapeutic intervention (27). Signaling via muscarinic receptors is critical for monocular deprivation-induced plasticity in the cat visual cortex (28, 29) and nucleus basalis-induced receptive field plasticity in rat primary auditory cortex (30, 31).
Muscarinic acetylcholine receptors (mAChR) are members of the large family of transmembrane, G-protein-coupled receptors that includes the rhodopsin family, odorant and taste receptors, and receptors for many neurotransmitters and neuromodulators (32). In vertebrates, there are five molecularly and possibly functionally distinct mAChR subtypes (33). Only a single mAChR has been identified in sequenced insect genomes, a bee brain EST database, and assembly 3.0 of the honey bee genome (34–36).
Muscarinic agonists and antagonists, defined on the basis of their actions on vertebrate receptors, are active at the insect muscarinic receptor (37–39). Binding of ligand to metabotropic receptors results in the activation of multiple intracellular pathways, including increases in inositol trisphosphate and diacylglycerol, inhibition of adenylyl cyclase, and a decrease in intracellular levels of cAMP (33). These changes, in turn, stimulate multiple downstream responses and can interact with signaling pathways coupled to other neurotransmitter receptors.
In the present study, we tested two hypotheses: first, that foraging experience causes an increase in the volume of the mushroom body neuropil; second, that cholinergic signaling via muscarinic receptors mediates the effect of foraging on the mushroom bodies. We tested the latter hypothesis by substituting pharmacological stimulation of muscarinic receptors for natural foraging experience.
We report here evidence that foraging does cause an increase in the volume of the mushroom body neuropil in honey bees and that experience of life in the hive, without flight from the hive, cannot substitute for the experience of foraging. We also demonstrate that an increase in the volume of the mushroom body neuropil can be induced by treatment of bees with the muscarinic agonist pilocarpine. Our finding of muscarinic receptor-mediated growth of the mushroom bodies reveals an evolutionarily conserved role for acetylcholine in the modulation of brain plasticity.
Methods
Animals. Honey bees were maintained at the University of Illinois Bee Research Facility by using standard apicultural techniques. Experiments were performed during the summers of 2002, 2003, and 2004. Experimental bees were derived from a mixture of European races (predominantly Apis mellifera ligustica). One-day-old adult worker bees (focal bees) were obtained by removing honeycombs containing pupae from large “source” colonies (each headed by a naturally mated queen) in the field and placing them in an incubator (34°C, 95% relative humidity). Bees that emerged over a 24-h period were marked with a spot of paint (Testor's PLA, Testor, Rockford, IL) on the thorax and introduced into an unrelated host colony.
Preliminary studies revealed that 10,000 focal bees were needed for each replicate of an experiment to ensure rapid recovery of sufficient sample sizes for all experimental groups. Because a typical queen lays no more than ≈1,500 eggs per day, we obtained bees from multiple source colonies over a 2-day period. Bees from different source colonies were mixed and assigned randomly to each experimental group.
Each host colony was housed in a 2-story Langstroth hive and contained a naturally mated queen, the 10,000 focal bees, and 30,000 unmarked background bees. One-week foragers were chosen for our experimental manipulations to ensure a focus on experience-dependent structural plasticity and to provide a bona fide experience enhancement, i.e., a second week of foraging. To increase recovery of one-week foragers and the probability that they would survive a full second week of foraging, we removed a portion of the host colony's nonfocal foraging force, causing most focal bees to initiate foraging a few days early (40).
Beginning when bees were 10 days of age, daily observations were made at the hive entrance for 6–8 h per day to identify bees that had initiated foraging. Foragers were identified according to standard criteria (bees returning to the hive with pollen loads, dusted with pollen, or abdomens distended with either nectar or water). One-day foragers identified at the hive entrance were marked with a second dot of paint and then allowed to reenter the hive and continue foraging for 1 week. Observations continued to reidentify these bees as one-week foragers. One-week foragers were either allowed to continue foraging in the field for a second week or were brought into the laboratory for further treatment.
Treatment. One-week foragers were placed in groups of 10–12 individuals in Plexiglas cages (10 × 10 × 7 cm) in a dark incubator (28°C at 95% relative humidity) for 1 week (Fig. 1). They were orally treated with one of the following cholinergic agents dissolved in 50% sucrose: pilocarpine hydrochloride (muscarinic agonist; Sigma P6503, 10–6 M and 10–8 M); scopolamine hydrochloride (muscarinic antagonist; Sigma S1013, 10–3 M), or nicotine (nicotinic agonist; Sigma N0267, 10–3 M). Doses were 10-fold lower than the LD50 we determined for each drug for bees housed under identical conditions (data not shown). Feeding tubes containing treatment solutions were changed daily (under red light, which is invisible to bees). Foragers cannot survive longer than 24 h without ingesting carbohydrates, so all surviving bees must have ingested the provided solution (41). Negative control (caged control) bees were housed under identical conditions but were fed only the 50% sucrose solution vehicle. Positive control bees were one-week foragers allowed to continue foraging under natural conditions in the field for a second week (two-week foragers). All treated and control bees were age-matched (median age 25 days within each trial). At the end of each experiment, bees were cold anesthetized (4°C). Dissection of the brain from the head capsule was completed within 4 h of chilling.
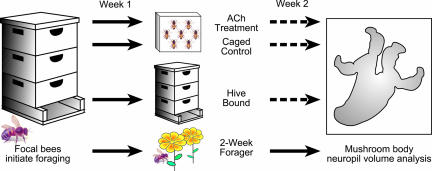
Fig. 1.
Caged bees in the laboratory. Caged bees were fed either a 50% sucrose solution (in water) or cholinergic agents dissolved in the sucrose solution. The volume of neuropil of the mushroom bodies was estimated in age-matched bees as a function of foraging experience and drug treatment.
Foraging Deprivation. One-week foragers (obtained as described above) were confined to their host colony by using a previously established technique in ref. 42. This confinement involved gluing a glass bead (1.5–2 mm high) on the dorsal surface of the thorax to increase the height of the thorax. A screen placed inside the hive prevented these bees from leaving the hive but were exposed to stimuli in the colony (i.e., nectar, pollen, wax, and their nestmates). The other bees from the same colony were allowed to come and go freely and obtain a second week of foraging experience.
Histology and Volume Analysis. Histology and regional volume estimation were performed with established methods in ref. 43. Brains dissected free from the head capsule were fixed by immersion in Bouin's alcoholic fixative, dehydrated, cleared, embedded in Paraplast wax, and sectioned transversely at 10 μm by using a rotary microtome. Complete sets of serial sections through each brain were stained with cresyl violet (Sigma C1791) and Luxol Fast Blue (Sigma S3382). The Cavalieri method was used to estimate the volume of the neuropil of the mushroom bodies (calyces, pedunculus, and lobes) and, in some groups, neuropils associated with the central complex. The central complex is a group of protocerebral neuropils implicated in motor control (44) and was studied here solely to explore the regional specificity of the mushroom body effects. Estimates of mushroom body neuropil volume were conducted blind and based on the Cavalieri protocol of systematic random sampling of one in every six sections. Honey bee workers are monomorphic, so no correction for body size is required. Volume estimates were made on one hemisphere of each brain, randomly selected. A BioQuant image analysis system was used to determine cross-sectional areas (BIOQUANT Image Analysis Corporation nova prime stereology toolkit, version 6.70.10).
Statistical Analysis. Estimates of the volume of the mushroom body neuropils were made for a total of 309 individuals, across nine independent experiments, conducted over three field seasons (Table 1). One-way ANOVA and Tukey-Kramer post hoc tests were performed to assess the differences in neuropil volumes between the treatment groups (systat version 10 for Windows, Systat). A QQ (quantile-quantile) plot was used to assure a normal distribution of the data. The correlation between flight experience and volume of the mushroom body neuropil was analyzed by using the Pearson test (SAS Institute 2001, sas proc glm). A broader analysis was performed to facilitate comparisons across all treatments and across all experiments. The % difference relative to the caged control group in each experiment was calculated by using a mixed ANOVA model and Dunnett post hoc test (sas proc mixed).
Table 1. Effects of foraging experience or muscarinic agonist on brain plasticity.
| Colony* | One-day forager | One-week forager | Caged control | Hive bound | Two-week forager | Pilo 10-6 M | Pilo 10-8 M | Nicotine | Pilo + Scop |
|---|---|---|---|---|---|---|---|---|---|
| A | 43.7 ± 1.0a (11) | 43.9 ± 1.0a (10) | 45.0 ± 1.3a (6) | — | 52.1 ± 1.3b (6) | — | — | — | — |
| B | — | — | 42.1 ± 1.2a (7) | 43.5 ± 1.2a (6) | 49.8 ± 1.1b (7) | — | — | — | — |
| C | — | — | 43.6 ± 1.3a (7) | 46.8 ± 1.1b (9) | 50.6 ± 1.3b (7) | — | — | — | — |
| D | — | — | 44.4 ± 1.2a (6) | 47.6 ± 1.2ab (6) | 50.4 ± 1.2b (6) | — | — | — | — |
| E | — | — | 44.3 ± 0.6a (12) | — | 47.9 ± 0.6b (14) | 48.9 ± 0.7b (8) | 45.5 ± 0.7a (10) | — | — |
| F | — | — | 43.4 ± 0.5a (16) | — | 49.7 ± 0.7b (11) | 49.2 ± 0.6b (13) | — | 45.4 ± 0.7a (10) | — |
| G | — | — | 43.8 ± 0.6a (10) | — | 49.4 ± 0.6b (12) | 48.0 ± 0.7b (7) | — | 44.6 ± 0.8a (6) | — |
| H | — | 43.6 ± 1.2a (8) | 45.1 ± 1.1a (9) | — | 52.1 ± 1.0b (11) | 50.6 ± 0.9b (13) | — | — | 43.9 ± 0.8a (15) |
| I | — | 42.6 ± 1.3a (8) | 44.6 ± 1.3a (8) | — | 52.8 ± 1.4b (7) | 50.5 ± 1.1b (10) | — | — | 43.3 ± 1.4a (7) |
| Model† estimate | 42.7 ± 1.1 (11) | 42.5 ± 0.7 (26) | 44.1 ± 0.5 (81) | 46.3 ± 0.8 (21)‡ | 50.4 ± 0.5 (81)§ | 49.6 ± 0.5 (41)§ | 46.4 ± 1.0 (10) | 45.7 ± 0.8 (16) | 42.8 ± 0.8 (22) |
Data are presented as mean volume (× 10-3 mm3) ± standard error. Number of brains for each comparison is given in parentheses. Lowercase letters indicate statistically distinguishable groups (Tukey post hoc analysis, P < 0.05). Pilo, pilocarpine; Scop, scopolamine.
Nine different colonies, one in each trial; each trial employed a subset of the various treatments.
Model estimate produced by GCLM, sas version 22. This value is the basis of calculations presented in Fig. 2.
P = 0.0328, Dunnett post hoc analysis.
P = 0.0001, Dunnett post hoc analysis.
Results
Effects of Foraging Experience on Mushroom Body Neuropil Volume. Daily observations at the entrance of hives in the field allowed us to identify previously marked bees of known age on the day they shifted from working in the hive to foraging. These one-day foragers were allowed to continue foraging under natural conditions for 1 week, at which time they were designated one-week foragers. One-week foragers were either allowed to continue foraging for a second week (two-week foragers) or deprived of a second week of foraging by confinement in cages in the laboratory for one week (caged controls; Figs. 1 and 2). The Cavalieri method was used to estimate the volume of the neuropil of the mushroom body in age-matched bees. There was a significant positive correlation between foraging experience and mushroom body neuropil volume (Pearson correlation coefficient = 0.622; P = 0.0005). Two-week foragers had significantly larger mushroom body neuropil volumes than one-day foragers (Tukey-Kramer; P = 0.0001) and one-week foragers (P = 0.0001). There was no difference in mushroom body neuropil volume between one-day foragers and one-week foragers (P = 0.998).
Fig. 2.
Control of foraging experience in honey bees. Extensive daily behavioral observations made at the entrance of hives in the field permitted identification of bees of known age on the day that they shifted from working in the hive to foraging. These one-day foragers were marked with a second paint dot and allowed to continue foraging (Week 1). Bees with 1 week of foraging experience were allowed to continue foraging in the field, confined to the hive, or confined in cages in the laboratory for 1 week and fed either sugar water or cholinergic agents dissolved in sugar water (Week 2). Dashed lines indicate bees prevented from foraging.
Caged bees with one previous week of foraging experience had significantly smaller mushroom body neuropil volumes than two-week foragers in nine of nine trials (each trial represents an independent, unrelated colony; in each trial, all groups of foragers were matched for age; Table 2, which is published as supporting information on the PNAS web site), conducted over three different field seasons (Table 1). Overall, two-week foragers showed a ≈14% increase in mushroom body neuropil volume relative to caged control bees. Because caged control bees did not show a significant difference in mushroom body neuropil volume compared with one-week foragers (Table 1), this robust effect reflects growth in the two-week foragers, rather than a decrease in volume as a result of laboratory caging for one week in the caged control bees. An overview of the differences among the key experimental groups, normalized to the mushroom body neuropil volume of the caged controls, is shown in Fig. 3.
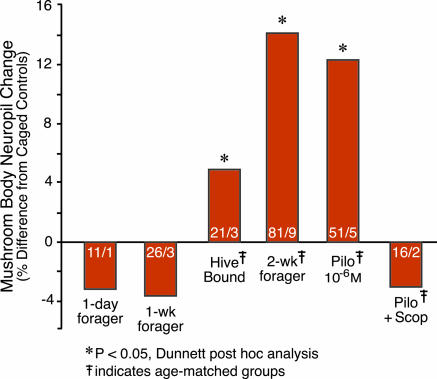
Fig. 3.
Effects of foraging experience or muscarinic agonist on the mushroom bodies. Estimates of the volume of the mushroom body neuropil were made for a total of 309 individuals, across nine experiments, conducted over three field seasons. To facilitate comparisons across all treatments, we calculated the % difference in neuropil volume relative to the caged control group in each experiment. Statistical analysis of these data used a mixed ANOVA model (F = 39.00; P = 0.0001; Table 1) and Dunnett post hoc tests (groups showing significant differences from the caged control group are indicated with the % difference). Key experimental groups are shown with the % difference indicated. Sample sizes are given in each bar (number of brains/number of trials). Complete data for these and all other groups are presented in Table 1.
Life in a beehive is complex, and bees in nature are exposed to diverse social and environmental stimuli in addition to those associated with foraging. Can the increase in mushroom body neuropil volume seen in two-week foragers relative to caged control bees be definitively ascribed to the effects of the second week of foraging, rather than to other experiences the foragers might possibly have had that the caged control bees did not? To answer this question, a group of one-week foragers was confined to their hive for the next week, and the volume of their mushroom body neuropil was compared to that of two-week foragers and caged control bees. These hive-bound bees had a significantly smaller volume of mushroom body neuropil than did two-week foragers (Table 1). Hive-bound bees actually had a volume of mushroom body neuropil intermediate between that found in two-week foragers and caged control bees, with an ≈5% increase in neuropil volume relative to caged control bees (Fig. 3). These results suggest that there are stimuli independent of foraging, such as social interactions or pheromonal cues, available to bees in a hive that are unavailable to bees in a laboratory cage that can affect the mushroom bodies. These results demonstrate a specific effect of foraging experience on mushroom body neuropil volume above and beyond the daily experience of life in the hive.
Pilocarpine Treatment Mimics the Effects of Extended Foraging Experience on Mushroom Body Neuropil Volume. Caged bees (one-week foragers; Fig. 1) treated orally with 10–6 M pilocarpine had a significantly larger volume of mushroom body neuropil compared with caged control bees (one-week foragers caged in the laboratory for a subsequent week) in five of five independent trials conducted over three different field seasons (Table 1). The magnitude of the increase was ≈13%, comparable to the magnitude of the mushroom body volume increase found in two-week foragers (Fig. 3). Mushroom body neuropil volumes for two-week foragers and pilocarpine-treated bees did not differ (Table 1).
The specificity of the effect of pilocarpine is indicated by the following results. First, a 100-fold lower dose of pilocarpine (10–8 M) had no effect on mushroom body neuropil volume (Table 1). Second, treatment of one-week foragers with nicotine (specific nicotinic receptor agonist) had no effect on mushroom body neuropil volume (Table 1). Third, concurrent treatment with pilocarpine (10–6 M) and scopolamine (10–4 M, muscarinic antagonist) had no effect on mushroom body neuropil volume (Table 1). Fourth, the effects of pilocarpine and foraging experience were apparently selective for the mushroom body, because there was no effect of either on the volume of other protocerebral neuropils (Fig. 4, which is published as supporting information on the PNAS web site). All trials in which these treatments failed to find an effect included a group treated with the 10–6 M dose of pilocarpine as a positive control, and in every trial, this treatment resulted in a significant increase in the volume of the mushroom body neuropil (Table 1). We conclude that treatment with 10–6 M pilocarpine reliably mimics the effects of a second week of foraging experience on the volume of the mushroom body neuropil in the bee brain.
Discussion
We have demonstrated that the experience of foraging increases the volume of the mushroom body neuropil in the honey bee. Previous studies have shown that the enlarged mushroom bodies characteristic of foragers relative to hive bees do not simply reflect aging, because comparable increases in mushroom body neuropil volume occur earlier in bees induced to forage precociously (8–10).
There are at least two components to the structural plasticity of the bee mushroom bodies. The first is an increase in volume that begins during metamorphosis, when the mushroom body neuropils first form, and continues into the early part of adult life (10, 42, 45, 46). This increase occurs in bees reared in social isolation in total darkness (42) and in bees prevented from flying outside of the hive (46). This first stage thus begins several weeks before the onset of foraging and appears to be experience-independent or, perhaps, anticipates the experience of foraging.
The second, experience-dependent, component of structural plasticity begins when foraging is initiated. It at least provides a brain record of accumulating foraging experience and may also contribute to improved foraging efficiency. We have successfully separated the experience-independent and experience-dependent components in the present study by focusing our experimental manipulations on the second week of foraging. Our results show that a second week of foraging under natural conditions induces more growth of the mushroom body neuropil than does a week in a laboratory cage or a week of hive confinement.
Our comparison of caged, hive-bound, and foraging bees allows us to begin to dissect the specific nature of the experiences that induce structural plasticity in the bee brain. As in vertebrates, the situation is complex (47). Foraging plays a dominant, but not exclusive, role in this process: Stimuli experienced in the hive can also induce structural plasticity in the bee brain, at least relative to the impoverished environment of a laboratory cage. Foraging itself conflates two major factors previously identified as important aspects of enrichment: voluntary motor behavior and the opportunity to learn new associations (in this case, the association of floral scents with food rewards) in a changing environment. It is not possible, on the basis of the present data, to dissociate the effects of exercise and learning on the mushroom bodies.
In light of the complex effects of experience on the structure of the nervous system, the results of our pilocarpine treatments are remarkably unambiguous. Chronic stimulation of muscarinic cholinergic receptors increased mushroom body neuropil volume in caged bees to a volume indistinguishable from that induced by a week of foraging experience.
Several earlier studies hinted at an association between stimulation of cholinergic receptors and learning mediated by the mushroom bodies. For example, treatment of bees with acetylcholinesterase inhibitors reduced the rate of habituation to an odor stimulus (48) and increased the number of correct responses on a standard test of olfactory association learning, the proboscis extension response (22). Application of muscarinic antagonists (atropine and scopolamine) to the mushroom bodies via the surface of the bee brain 20 min after a learning trial significantly reduced performance of the conditioned response (proboscis extension) at 10 and 20 min after injection but not at later times (39, 49). Expression of the acetylcholinesterase gene by the Kenyon cells of the mushroom bodies is reduced in the brains of foragers relative to the brains of bees working in the hive (22), a finding that suggests that degradation of acetylcholine is attenuated in foragers. Chronic administration of a muscarinic agonist such as pilocarpine may mimic this foraging-associated enhancement of cholinergic neurotransmission.
A model for how cholinergic signaling mediates brain plasticity has emerged, in part, from studies of the vertebrate primary auditory cortex. Neurons in this brain region that are exposed to an auditory signal under conditions of muscarinic inhibition failed to show the expected shifts of frequency tuning, suggesting that muscarinic signaling is required to allow these neurons to respond adaptively to experience (31). Muscarinic signaling may serve a comparable permissive role in the honey bee, allowing the Kenyon cells to respond to other inputs with increases in dendritic length and branching. But because pharmacological stimulation of muscarinic receptors resulted in the continued growth of the mushroom body neuropil in one-week foragers completely denied further foraging experience, it is also possible to argue that, from the perspective of the mushroom bodies, foraging experience itself is encoded as activation of muscarinic receptors.
A detailed understanding of the cell and molecular mechanisms that couple experience to brain plasticity has not yet been achieved for any system, even those studied as intensively as regulation of the developing visual cortex of mammals (50). Our results suggest that the bee can be used to discover how intracellular second messenger pathways stimulated by muscarinic receptor activation induce changes in neuronal gene expression that result in experience-dependent structural brain plasticity. This knowledge is likely to have general significance, given our finding of an evolutionarily conserved role for acetylcholine in the mediation of brain plasticity.
Supplementary Material
Acknowledgments
We thank K. Pruiett, D. A. Rauch, and A. L. Stetler for assistance in the field and D. F. Clayton, P. E. Gold, C. Grosman, J. M. Juraska, and members of the Robinson and Fahrbach laboratories for helpful discussions and comments on the manuscript. This work was supported by a National Institutes of Health (NIH) Developmental Psychobiology and Neurobiology Training Grant Fellowship (to N.I.; W. T. Greenough, principal investigator) and grants from the National Science Foundation (to S.E.F. and G.E.R.), NIH (to G.E.R.), and Burroughs Wellcome Fund (to G.E.R.).
Author contributions: N.I., G.E.R., and S.E.F. designed research; N.I. performed research; N.I. analyzed data; and N.I., G.E.R., and S.E.F. wrote the paper.
Conflict of interest statement: No conflicts declared.
References
- 1.Greenough, W. T. & Volkmar, F. R. (1973) Exp. Neurol. 40, 491–504. [DOI] [PubMed] [Google Scholar]
- 2.Clayton, N. S. & Krebs, J. R. (1994) Proc. Natl. Acad. Sci. USA 91, 7410–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeley, T. D. (1982) Behav. Ecol. Sociobiol. 11, 287–293. [Google Scholar]
- 4.Neukirch, A. (1982) J. Comp. Physiol. B 146, 35–40. [Google Scholar]
- 5.Menzel, R. (1985) in Experimental Behavioral Ecology and Sociobiology, eds. Hölldobler, B. & Lindauer, M. (Fischer, New York).
- 6.Menzel, R., Geiger, K., Chittka, L., Joerges, J., Kunze, J & Müller, U (1996) J. Exp. Biol. 199, 141–146. [DOI] [PubMed] [Google Scholar]
- 7.Winston, M. L. (1987) The Biology of the Honey Bee (Harvard Univ. Press, Cambridge, MA).
- 8.Withers, G. S., Fahrbach, S. E. & Robinson, G. E. (1993) Nature 364, 238–240. [DOI] [PubMed] [Google Scholar]
- 9.Durst, C., Eichmüller, S. & Menzel, R. (1994) Behav. Neural Biol. 62, 259–263. [DOI] [PubMed] [Google Scholar]
- 10.Farris, S. M., Robinson, G. E. & Fahrbach, S. E. (2001) J. Neurosci. 21, 6395–6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dukas, R. & Visscher, P. K. (1994) Anim. Behav. 48, 1007–1012. [Google Scholar]
- 12.Farris, S. M. & Sinakevitch, I. (2003) Arthropod Struct. Dev. 32, 79–101. [DOI] [PubMed] [Google Scholar]
- 13.Dubnau, J. & Tully, T. (2001) Curr. Biol. 11, R240–R243. [DOI] [PubMed] [Google Scholar]
- 14.Roman, G. & Davis, R. L. (2001) BioEssays 23, 571–581. [DOI] [PubMed] [Google Scholar]
- 15.Gerber, B., Tanimoto, H. & Heisenberg, M. (2004) Curr. Opin. Neurobiol. 14, 737–744. [DOI] [PubMed] [Google Scholar]
- 16.Davis, R. L. (2005) Annu. Rev. Neurosci. 28, 275–302. [DOI] [PubMed] [Google Scholar]
- 17.Xia, S., Miyashita, T., Fu, T. F., Lin, W. Y., Wu, C. L., Pyzocha, L., Lin, I. R., Saitoe, M., Tully, T. & Chiang, A. S. (2005) Curr. Biol. 15, 603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahrbach, S. E., Strande, J. L. & Robinson, G. E. (1995) Neurosci. Lett. 197, 145–148. [DOI] [PubMed] [Google Scholar]
- 19.Cayre, M., Strambi, C., Charpin, P., Augier, R., Meyer, M. R., Edwards, J. S. & Strambi, A. (1996) J. Comp. Neurol. 371, 300–310. [DOI] [PubMed] [Google Scholar]
- 20.Gronenberg, W. (2001) J. Comp. Neurol. 435, 474–489. [DOI] [PubMed] [Google Scholar]
- 21.Hansson, B. S. & Anton, S. (2000) Annu. Rev. Entomol. 45, 203–231. [DOI] [PubMed] [Google Scholar]
- 22.Shapira, M., Thompson, C. K., Soreq, H. & Robinson, G. E. (2001) J. Mol. Neurosci. 17, 1–12. [DOI] [PubMed] [Google Scholar]
- 23.Huang, Z.-Y. & Knowles, C. O. (1990) Comp. Biochem. Physiol. C 97, 275–281. [Google Scholar]
- 24.Onai, T., FitzGerald, M. G., Arakawa, S., Gocayne, J. D., Urquhart, D. A., Hall, L. M., Fraser, C. M., McCombie, W. R. & Venter, J. C. (1989) FEBS Lett. 255, 219–225. [DOI] [PubMed] [Google Scholar]
- 25.Gold, P. E. (2003) Neurobiol. Learn. Mem. 80, 194–210. [DOI] [PubMed] [Google Scholar]
- 26.Mesulam, M. (2004) Learn. Mem. 11, 43–49. [DOI] [PubMed] [Google Scholar]
- 27.Gsell, W., Jungkunz, G. & Riederer, P. (2004) Curr. Pharm. Des. 10, 265–293. [DOI] [PubMed] [Google Scholar]
- 28.Gu, Q. & Singer, W. (1993) Eur. J. Neurosci. 5, 475–485. [DOI] [PubMed] [Google Scholar]
- 29.Gu, Q. (2003) Neurobiol. Learn. Mem. 80, 291–301. [DOI] [PubMed] [Google Scholar]
- 30.Miasnikov, A. A., McLin, D., III, & Weinberger, N. M. (2001) NeuroReport 12, 1537–1542. [DOI] [PubMed] [Google Scholar]
- 31.Weinberger, N. M. (2004) Nat. Rev. Neurosci. 5, 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brody, T. & Cravchik, A. (2000) J. Cell Biol. 150, F83–F88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss, J. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 423–450. [DOI] [PubMed] [Google Scholar]
- 34.Adams, M. D., Celniker, S. E., Holt, R. A., Evans, C. A., Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P. W., Hoskins, R. A., Galle, R. F., et al. (2000) Science 287, 2185–2195. [DOI] [PubMed] [Google Scholar]
- 35.Holt, R. A., Subramanian, G. M., Halpern, A., Sutton, G. G., Charlab, R., Nusskern, D. R., Wincker, P., Clark, A. G., Ribeiro, J. M., Wides, R., et al. (2002) Science 298, 129–149.12364791 [Google Scholar]
- 36.Whitfield, C. W., Band, M. R., Bonaldo, M. F., Kumar, C. G., Liu, L., Pardinas, J. R., Robertson, H. M., Soares, M. B., Robinson, G. E. (2002) Genome Res. 12, 555–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trimmer, B. A. & Weeks, J. C. (1989) J. Exp. Biol. 144, 303–337. [Google Scholar]
- 38.Johnston, R. M. & Levine, R. B. (1996) J. Neurophysiol. 76, 3178–3195. [DOI] [PubMed] [Google Scholar]
- 39.Lozano, V. C. & Gauthier, M. (1998) Pharmacol. Biochem. Behav. 59, 903–907. [DOI] [PubMed] [Google Scholar]
- 40.Huang, Z.-Y. & Robinson, G. E. (1996) Behav. Ecol. Sociobiol. 39, 147–158. [Google Scholar]
- 41.Lorenz, M. W., Kellner, R., Volkl, W., Hoffman, K. H. & Woodring, J. (2001) J. Insect Physiol. 47, 563–571. [DOI] [PubMed] [Google Scholar]
- 42.Fahrbach, S. E., Moore, D., Capaldi, E. A., Farris, S. M. & Robinson, G. E. (1998) Learn. Mem. 5, 115–123. [PMC free article] [PubMed] [Google Scholar]
- 43.Fahrbach, S. E., Giray, T. & Robinson, G. E. (1995) Neurobiol. Learn. Mem. 63, 181–191. [DOI] [PubMed] [Google Scholar]
- 44.Ilius, M., Wolf, R. & Heisenberg, M. (1994) J. Neurogenet. 9, 189–206. [DOI] [PubMed] [Google Scholar]
- 45.Farris, S. M., Robinson, G. E., Davis, R. L. & Fahrbach, S.E. (1999) J. Comp. Neurol. 414, 97–113. [DOI] [PubMed] [Google Scholar]
- 46.Withers, G. S., Fahrbach, S. E. & Robinson, G. E. (1995) J. Neurobiol. 26, 130–144. [DOI] [PubMed] [Google Scholar]
- 47.van Praag, H., Kempermann, G. & Gage, F. H. (2000) Nat. Rev. Neurosci. 1, 191–198. [DOI] [PubMed] [Google Scholar]
- 48.Braun, G. & Bicker, G. (1992) J. Neurophysiol. 67, 588–598. [DOI] [PubMed] [Google Scholar]
- 49.Gauthier, M., Lozano, V. C., Zaoujal, A. & Richard, D. (1994) Behav. Brain Res. 63, 145–149. [DOI] [PubMed] [Google Scholar]
- 50.Berardi, N., Pizzorusso, T., Ratto, G. M. & Maffei, L. (2003) Trends Neurosci. 26, 369–378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.