Abstract
Heterotrimeric G proteinshave been previously linked to plant defense; however a role for the Gβγ dimer in defense signaling has not been described to date. Using available Arabidopsis (Arabidopsis thaliana) mutants lacking functional Gα or Gβ subunits, we show that defense against the necrotrophic pathogens Alternaria brassicicola and Fusarium oxysporum is impaired in Gβ-deficient mutants while Gα-deficient mutants show slightly increased resistance compared to wild-type Columbia ecotype plants. In contrast, responses to virulent (DC3000) and avirulent (JL1065) strains of Pseudomonas syringae appear to be independent of heterotrimeric G proteins. The induction of a number of defense-related genes in Gβ-deficient mutants were severely reduced in response to A. brassicicola infection. In addition, Gβ-deficient mutants exhibit decreased sensitivity to a number of methyl jasmonate-induced responses such as induction of the plant defensin gene PDF1.2, inhibition of root elongation, seed germination, and growth of plants in sublethal concentrations of methyl jasmonate. In all cases, the behavior of the Gα-deficient mutants is coherent with the classic heterotrimeric mechanism of action, indicating that jasmonic acid signaling is influenced by the Gβγ functional subunit but not by Gα. We hypothesize that Gβγ acts as a direct or indirect enhancer of the jasmonate signaling pathway in plants.
Heterotrimeric G proteins (G proteins) are an integral component in a plethora of signal transduction pathways mediating the action of a family of seven transmembrane receptors known as G protein-coupled receptors. The canonical G-protein heterotrimer consists of three different subunits (Gα, Gβ, and Gγ) and has been found in all eukaryotes from yeast (Saccharomyces cerevisiae) and slime molds (Dictyostelium discoideum) to higher plants and mammals. In plants, one canonical Gα, one Gβ, and two Gγ subunits have been identified (Ma, 1994; Weiss et al., 1994; Mason and Botella, 2000, 2001). Initial pharmacological studies associated G proteins with a wide range of signal transduction processes in plant growth and development; however, only recently genetic evidence has been provided using mutants lacking either the Gα or Gβ subunits. It is now well established that G proteins are involved in processes such as auxin-related cell division (Ullah et al., 2003), GA3- and brassinosteroid-controlled seed germination (Ullah et al., 2002), abscisic acid (ABA) stimulation of phospholipase D in barley (Hordeum vulgare) aleurone (Ritchie and Gilroy, 2000), stomata function in Arabidopsis (Arabidopsis thaliana; Fan et al., 2004), responsiveness to low concentrations of GA3 in rice (Oryza sativa; Ueguchi-Tanaka et al., 2000), and resistance to rice blast in rice (Suharsono et al., 2002). Surprisingly, extensive phenotypic analyses of Arabidopsis Gα and Gβ mutants have revealed minor morphological differences between the mutants and wild-type plants (Lease et al., 2001; Ullah et al., 2001, 2003; Wang et al., 2001).
G proteins have been implicated in plant defense, although the existing research has predominantly relied upon the use of synthetic pharmacological agents and cell cultures (Legendre et al., 1992; Beffa et al., 1995; Gelli et al., 1997). Until very recently, the only evidence that disease response is affected in a G-protein mutant had been reported in the Daikoku d1 rice mutants. Several alleles of these mutants exist, and it has been shown that the mutation is the result of a knockout of the Gα subunit (Ashikari et al., 1999). Two reports (Suharsono et al., 2002; Komatsu et al., 2004) have provided evidence that the d1 mutants have impaired resistance to infection by pathogens and delayed activation of pathogenesis-related (PR) gene expression in leaves infected by an avirulent race of rice blast fungus. Although the d1 mutants were ultimately resistant to the fungus, disease symptoms in the mutant were found to develop earlier than in the wild type. While this manuscript was being prepared, a report has shown that ERECTA receptor-like kinase and G proteins are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina in Arabidopsis (Llorente et al., 2005).
In this article, we present genetic evidence that G proteins play an important role in plant defense against necrotrophic fungi. We show that Gβγ- but not Gα-mediated signaling is involved in the defense against necrotrophic pathogens. We also demonstrate that lack of Gβγ signaling affects a number of jasmonic acid (JA)-mediated responses. To our knowledge, this relationship between JA and G-protein signaling pathways has not been proven before. We hypothesize that G proteins might be involved in plant defense against necrotrophic fungi through enhancement of the JA signaling pathway.
RESULTS
Resistance/Susceptibility of Arabidopsis against Pseudomonas syringae Is Independent of G Proteins
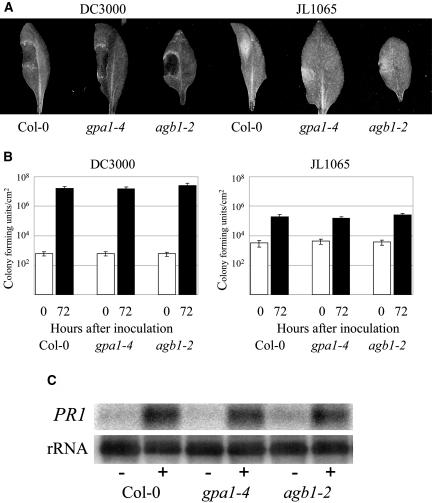
Two previously characterized mutant lines lacking the Gα subunit (gpa1-3 and gpa1-4; Jones et al., 2003) and two lacking a functional Gβ subunit (agb1-1 and agb1-2; Lease et al., 2001; Ullah et al., 2003) were challenged with Pseudomonas syringae pv tomato strains DC3000 (compatible interaction with Columbia [Col-0]) and JL1065 (incompatible interaction with Col-0). A number of experiments were performed using a series of inoculum concentrations between 105 and 108 colony forming units (cfu)/mL. Disease progression was monitored through observations of chlorosis and necrosis development in the compatible interaction, whereas the incompatible interaction displayed small lesions resulting from the hypersensitive response, as described by Dong et al. (1991). Figure 1A shows the characteristic lesion development in leaves 72 h after inoculation. No noticeable differences were observed in any of the mutant lines assayed with respect to control wild-type lines (Col-0) at all tested concentrations (only gpa1-4 and agb1-2 lines are shown in the figure although identical results were observed for the remaining mutant lines). In planta pathogen growth was measured by determining bacterial density (cfu/cm2) in infected leaves 72 h after inoculation. Colony counts again showed no differences between the mutant and control lines in either the compatible or the incompatible interactions (Fig. 1B).
Figure 1.
Analysis of Arabidopsis Col-0, Gα-deficient (gpa1-4), and Gβ-deficient (agb1-2) mutants in response to infection with P. syringae pv tomato virulent DC3000 and avirulent JL1065 strains. A, Characteristic lesion development 72 h after inoculation with 108 cfu/mL. B, In planta bacterial growth from inoculated leaves at 0 and 72 h post inoculation with 105 cfu/mL. Shown are mean values of three independent experiments. Error bars represent se. C, PR1 gene induction 48 h after inoculation with 108 cfu/mL JL1065; (−) denotes mock-inoculated samples, (+) denotes JL1065 inoculations. Total RNA was extracted, separated by gel electrophoresis, and transferred to a nylon membrane before hybridization with the PR1 probe (At2g14610). Finally, the membrane was hybridized with a ribosomal probe to prove equal loading in all lanes.
The salicylic acid (SA) pathway plays an essential role in the plant's defense response to Pseudomonas pathogens (Thomma et al., 2001). We therefore examined the expression of PR1 (At2g14610), a gene controlled by SA and strongly induced after inoculation with avirulent P. syringae strains (Thomma et al., 2001). PR1 mRNA levels 48 h after inoculation with P. syringae JL1065 were strongly induced, compared with mock-inoculated samples, and no differences were observed between mutant and wild-type lines (Fig. 1C).
Arabidopsis Resistance to Fusarium oxysporum and Alternaria brassicicola Is Compromised in Gβ-Deficient But Not in Gα-Deficient Mutants
Fusarium oxysporum (f. sp. conglutinans) is a soil-borne necrotrophic fungus that penetrates plants through the root tip, secondary root formation points, and wounds, and subsequently colonizes the plant through the vascular system. Typical disease symptoms of Fusarium infection in Arabidopsis are the appearance of chlorosis in leaves and the retardation in plant growth, which ultimately results in the death of the plant (Mauchmani and Slusarenko, 1994; Agrios, 2005). Two-week-old wild-type (Col-0) plants (4–6 leaf stage) as well as gpa1-3, gpa1-4, agb1-1, and agb1-2 mutants were inoculated with F. oxysporum.
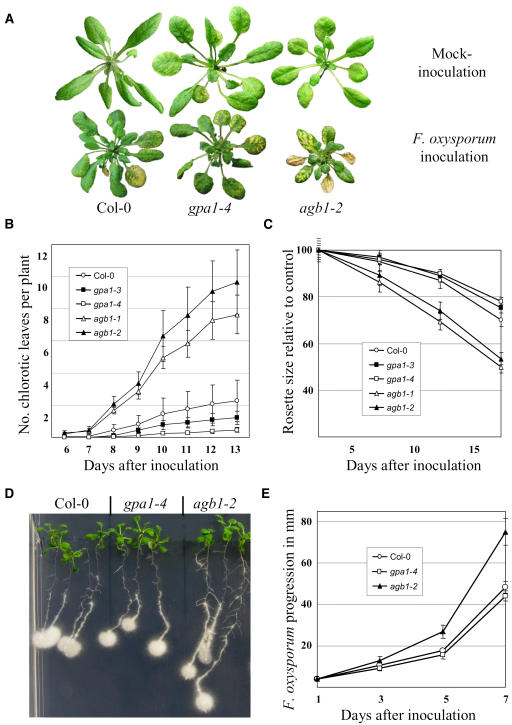
The first disease symptoms, manifested as yellow chlorosis in leaf veins, were observed 6 to 9 d after inoculation and, as disease progressed, there were clear developmental differences between Fusarium-infected and their respective control mock-inoculated plants. When infected plants were visually inspected, it was obvious that Gβ mutants were more severely affected than wild-type and Gα mutants (Fig. 2A). The number of chlorotic leaves per plant was recorded every day from the appearance of the first diseased leaf during the experiment (Fig. 2B). Both Gβ mutants, agb1-1 and agb1-2, developed a chlorotic pattern considerably earlier than wild-type plants (P < 0.01), while both Gα mutants were slightly delayed compared to wild type (Col-0). This delay was statistically significant for gpa1-4 (P < 0.05), while for gpa1-3 the difference was not statistically significant. This experiment was repeated four times with similar results. For all experiments, statistical significance was determined using the Student's t test.
Figure 2.
Differential response of wild-type, Gα-, and Gβ-deficient mutants to F. oxysporum. A, Characteristic disease symptoms: leaf chlorosis and impaired vegetative development 10 d after inoculation. B, Rate of appearance of diseased leaves after F. oxysporum root inoculation. Approximately 100 2-week-old plants of each genotype were evaluated. Error bars represent se. C, Inhibition of rosette growth after F. oxysporum root inoculation expressed relative to the mean growth of the same genotype after mock inoculation. Mean values and corresponding ses calculated from approximately 100 inoculated and 50 mock-inoculated plants for each genotype. D, Progression of F. oxysporum along Arabidopsis roots, 5 d after inoculation. E, Average rate of fungus expansion expressed in millimeters per day calculated on at least 30 seedlings per genotype. Error bars represent se.
Vegetative growth was also impaired to different degrees in wild-type plants and the mutants infected with F. oxysporum. The rosette diameter of F. oxysporum and mock-inoculated plants was measured at 5, 10, and 15 d after inoculation. Figure 2C shows the inhibition of rosette growth expressed as the relative size of Fusarium-inoculated plants versus mock-inoculated plants of the same genotype. Growth of both Gβ mutants was significantly affected by the pathogen 5 d after inoculation (P < 0.05) while Gα and wild-type plants were almost indistinguishable from their respective controls. By day 15, the size of the Fusarium-infected Gβ mutants was half of their controls while wild-type and gpa1 mutants were clearly less affected (70%–80% size of the control plants). Absolute values (day 15) for the mean rosette diameter of mock-inoculated wild-type (Col-0), gpa1-3, gpa1-4, agb1-1, and agb1-2 plants were 69.7 ± 7.1, 74.1 ± 12.3, 72.3 ± 11.1, 58.6 ± 7.0, and 57.4 ± 8.5 mm, respectively (shown as averages ± se), while leaves inoculated with F. oxysporum displayed measurements of 51.9 ± 7.7, 55.9 ± 8.5, 56.5 ± 9.2, 29.3 ± 5.3, and 30.7 ± 5.6 mm, respectively.
The rate of fungal progression along the roots was measured after applying one drop (2 μL) of F. oxysporum microconidia (106/mL) onto the root tips of 10-d-old plants grown on standard Murashige and Skoog (MS) medium. The fungus grew preferentially along the roots rather than radially on the agar. Figure 2D shows that F. oxysporum colonized Gβ mutant roots faster than wild-type (Col-0) and Gα mutants. The average rate of F. oxysporum colonization of the roots expressed in millimeters per day was measured and found to be significantly higher (P < 0.01) in agb1 mutants (Fig. 2E).
Pretreatment with methyl jasmonate (MeJA) effectively reduces necrotroph-induced disease development in wild-type plants as well as a number of mutants, but not in the MeJA-insensitive coronatine insensitive 1 (Vijayan et al., 1998; Thomma et al., 2000). We performed experiments pretreating agb1-1 and agb1-2 mutants with MeJA before infection with F. oxysporum. In our hands, pretreatment did not enhance the resistance to the fungus in any of the lines (data not shown).
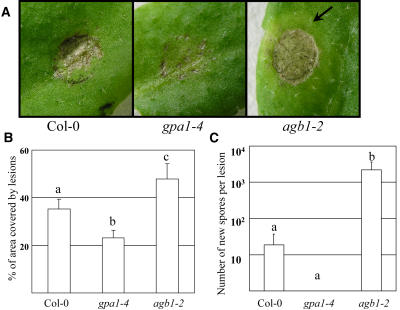
In contrast to F. oxysporum, Alternaria brassicicola is an air-borne avirulent pathogen of Arabidopsis ecotype Col-0 (Penninckx et al., 1996; Schenk et al., 2000; Thomma et al., 2000; Schenk et al., 2003; van Wees et al., 2003), even though it has been reported that certain isolates can reproduce at a very low rate under favorable conditions (van Wees et al., 2003). Application of a droplet of water with A. brassicicola spores (106 spores/mL) on the leaf surface caused small necrotic lesions that were clearly different in wild type and mutants (Fig. 3A). Gβ-deficient mutants frequently displayed necrotic lesions covering almost 100% of the inoculated area, which was not observed in wild-type or Gα-deficient mutants. To quantify this response to A. brassicicola, we measured the necrotic lesion area (given as a percentage of the droplet-inoculated area) by quantification of pixels from digital photographs. Figure 3B shows that in agb1-1 and agb1-2 mutants, 48% of the inoculated area developed into necrotic tissue compared to an average of 35% for wild-type plants and 23% for Gα mutants. Approximately 2 d after necrotic lesion development, a yellow chlorotic halo was often observed around the lesions in agb1-1 and agb1-2 mutants (Fig. 3A). This type of chlorosis is typical of A. brassicicola infection in compatible hosts (Conn et al., 1988; Kagan and Hammerschmidt, 2002). In addition, some of the lesions on the Gβ mutants started to spread after 3 to 5 d, while no spread was ever observed in wild-type or Gα mutants. It has been previously shown for Arabidopsis-A. brassicicola interactions that spreading lesions are associated with the fungus' ability to grow and produce new spores (van Wees et al., 2003); therefore, we quantified the number of newly developed fungal spores as described by van Wees et al. (2003; Fig. 3C). New A. brassicicola spores were never observed in Gα mutants, while wild-type plants contained negligible amounts (average 18.5 per lesion). In contrast, leaves of Gβ mutants displaying large spreading lesions contained a substantial number (average 2,203 per lesion) of newly formed spores. Lesion expansion and the production of a substantial number of new spores at the inoculation site indicate that the resistance to A. brassicicola is compromised in Gβ but not in Gα mutants.
Figure 3.
Differential response of wild-type, Gα-, and Gβ-deficient mutants to A. brassicicola. A, Maximal nonspreading lesions 3 d after inoculation. The chlorotic halo typical of A. brassicicola infection in compatible hosts is indicated with an arrow. B, Quantitative estimation of lesion development. The original droplet area was set as 100% and the area covered by necrotic tissue was calculated as a percentage of the droplet area. Data points represent averages with ses of at least 30 lesions for each genotype. Letters indicate statistically significant differences between genotypes (Student's t test, P < 0.05, n > 20). C, Average number of de novo formed spores per lesion after inoculation with A. brassicicola. Data points represent averages with ses of measurements on the six most developed lesions 8 d after inoculation (logarithmic scale). Letters indicate statistically significant differences between genotypes (Student's t test, P < 0.05, n > 6).
To provide additional information about the role of each individual functional subunit in disease resistance, we examined the behavior of a double-null mutant for GPA1 and AGB1 in response to infection with F. oxysporum and A. brassicicola. Disease progression was examined in the double gpa1-4/agb1-2 mutant alongside with wild type (Col-0) and each of the individual subunit mutants, gpa1-4 and agb1-2, after inoculation with F. oxysporum scoring the appearance of chlorotic diseased leaves. In our experiments, disease progression in the double mutant was indistinguishable from agb1-2 plants. Fifteen days after F. oxysporum inoculation, double mutants had 8.1 ± 2.5 diseased leaves per plant on average versus 2.3 ± 0.8, 0.9 ± 0.4, and 9.4 ± 1.6 observed in wild-type, gpa1-4, and agb1-2 plants, respectively. Lesion development after A. brassicicola inoculation revealed a very similar pattern with double gpa1-4/agb1-2 mutant plants being indistinguishable from agb1-2 plants and showing increased lesion severity compared to Col-0 control plants. As previously observed, gpa1-4 plants showed slightly less severity than Col-0.
Gβ-Deficient Mutants Display Reduced Induction Levels of Defense-Related Genes upon A. brassicicola Infection and MeJA Treatment
To test whether gene expression is altered in G-protein mutants, we selected four genes previously used as markers of defense-related processes and signaling pathways: GST1 (At1g02930, encoding glutathione S-transferase, an oxidative burst reporter gene; Marrs, 1996; Grant et al., 2000), PDF1.2 (At5g44420, encoding a plant defensin; Penninckx et al., 1998), OPR1 (At1g76680, encoding 12-oxophytodienoate reductase, involved in JA biosynthesis; Biesgen and Weiler, 1999), and PAD3 (At3g26830, encoding cytochrome P450 monooxygenase, involved in camalexin biosynthesis, also known as CYP71B15; Zhou et al., 1999). The choice of A. brassicicola over F. oxysporum for our experiments was based on three reasons. First, as we have shown above, the resistance against A. brassicicola was compromised in Gβ mutants. Second, since A. brassicicola infects aerial tissue and disease symptoms appear no earlier than 48 h after inoculation, it is possible to study primary or direct gene induction events before the disease symptoms appear. As the disease progresses in later stages of the infection, secondary processes can affect the expression of the selected genes. Third, the interaction between Arabidopsis and A. brassicicola has been extensively studied and a number of genes induced during infection have been previously reported (Schenk et al., 2000, 2003; van Wees et al., 2003).
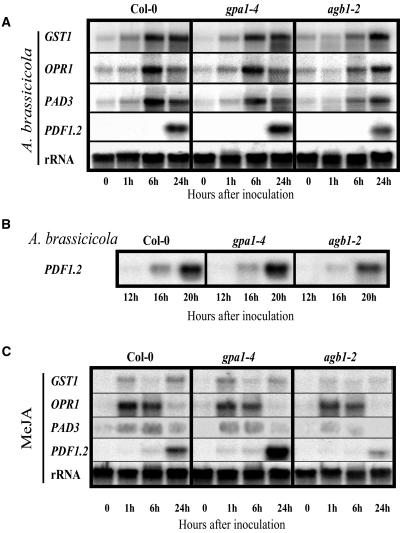
Three-week-old wild-type, agb1-2, and gpa1-4 plants were transferred into a growth cabinet with 100% humidity 24 h before inoculation with A. brassicicola spores. In agreement with previously reported data (van Wees et al., 2003), all four genes were induced in wild-type (Col-0) plants (Fig. 4A), but while gpa1-4 plants showed very similar behavior to wild type, agb1-2 mutants displayed lower and/or delayed induction of the studied genes. In particular, GST1, OPR1, and PAD3 induction patterns were almost identical in wild-type and gpa1-4 mutants and obviously delayed in agb1-2 mutants, indicating a delayed response of Gβ-deficient plants to the infection. PDF1.2 levels were noticeably lower in agb1-2 plants compared to wild type (Col-0), while expression levels were higher in gpa1-4 plants. To provide a more detailed PDF1.2 gene induction profile between 6 and 24 h, we performed an additional experiment sampling every 4 h after A. brassicicola infection. Figure 4, A and B, shows that PDF1.2 induction is clearly reduced in Gβ-deficient mutants.
Figure 4.
Expression of defense-related genes in response to infection with A. brassicicola or MeJA treatment. Three-week-old wild-type plants, Gα-, and Gβ-deficient mutants were infected with A. brassicicola (A and B), or treated with 50 μm MeJA (C). RNA blots were hybridized with the probes indicated on the left. The experiments were repeated twice with the same results.
It is well established that JA, rather than ethylene or SA signaling, is a major pathway involved in the resistance of Arabidopsis to A. brassicicola (Penninckx et al., 1998; Thomma et al., 1998, 1999a, 1999b; van Wees et al., 2003). To investigate whether the JA signaling pathway is affected in G-protein mutants, we compared the response of wild-type Gα- and Gβ-deficient plants to exogenous MeJA. Three-week-old plants were placed in sealed containers with high humidity for 24 h prior to spraying with 50 μm MeJA. Leaf tissue was harvested 0, 1, 6, and 24 h after the treatments and expression levels analyzed by northern-blot hybridization (Fig. 4C).
The differences in PDF1.2 expression levels observed among the three genotypes in response to A. brassicicola infection were even more dramatic when plants were treated with MeJA alone. agb1-2 mutant plants clearly show lower levels of induction than wild type and gpa1-4 plants show higher levels than wild type (compare Fig. 4, A and C). The behavior of the remaining three genes was also coherent with the observations made after A. brassicicola infection with the agb1-2 mutant showing decreased levels when compared to wild-type and gpa1-4 plants.
Gβ Deficiency Affects a Number of MeJA-Induced Responses
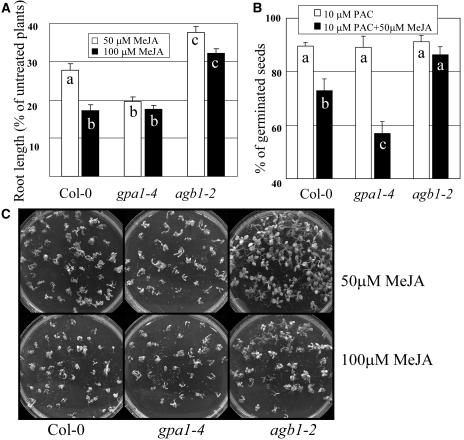
To further study the relationship between G proteins and JA signaling outside the plant defense pathways, we subjected wild-type (Col-0) control and mutant plants to a series of specific assays to study their response to MeJA treatment (Fig. 5).
Figure 5.
Differential responses of wild type, Gα-, and Gβ-deficient mutants to exogenous MeJA treatment. A, Seedlings were grown for 14 d on 1× MS, 2% Suc plates supplemented with 50 μm and 100 μm of MeJA, or without MeJA. Roots of at least 100 seedlings of each genotype were measured for each treatment. Data is presented as a percentage of the lengths of treated roots compared to their respective nontreated controls. Data points represent averages with ses. Letters indicate statistically significant differences between genotypes (Student's t test, P < 0.05, n > 100). B, Germination percentages of at least 120 seeds pretreated with 10 μm PAC and sown on 1× MS and 1% Suc plates with or without 50 μm MeJA. Germination was assessed 2 d after transferring plates to a 23°C incubator under continuous light. Data points represent averages with ses of three experiments. Letters indicate statistically significant differences between genotypes (Student's t test, P < 0.05, n = 3). C, Seedlings were grown on MS plates supplemented with 50 μm or 100 μm MeJA and photographed after 20 d.
A well-known response to MeJA treatment is inhibition of root elongation (Staswick et al., 1992). Roots of over 100 2-week-old wild-type (Col-0), gpa1-4, and agb1-2 seedlings grown on plates containing 0, 50, or 100 μm MeJA were measured. All seedlings showed inhibition of root elongation by MeJA treatment when compared to their respective nontreated genotypes, but the effect was significantly less severe in agb1-2 mutants (P < 0.01) and significantly more severe in gpa1-4 mutants (P < 0.01) compared to wild type (Fig. 5A).
We also tested the overall sensitivity of the mutants to elevated concentrations of MeJA during vegetative development. The growth of wild-type and Gα mutants in plates containing 50 μm MeJA was clearly impaired with a high rate of seedling mortality observed, while Gβ mutants were less affected and no mortality observed (Fig. 5C). When the concentration of MeJA was raised to 100 μm, Gβ mutants showed some seedling death, indicating once again that although MeJA sensitivity is impaired in those plants, they are not completely insensitive to this hormone. At the same MeJA concentration, wild-type and Gα mutants were clearly more affected than Gβ mutants with a large proportion of dead plants.
Inhibition of seed germination by MeJA has been documented for some plant species (Wilen et al., 1991; Preston et al., 2002). In Arabidopsis, the effect of MeJA in concentrations as high as 100 μm is rather minor; however, addition of only 2 μm of ABA had a strong synergistic effect on germination inhibition by MeJA (Staswick et al., 1992). When wild-type (Col-0), gpa1-4, and agb1-2 mutants were treated with 2 μm of ABA plus 50 μm MeJA, seed germination was suppressed by 13.0%, 38.4%, and 29.9%, respectively. At the same time, germination rates on control plates (no hormones) or with individual hormone treatments, either 2 μm ABA or 50 μm MeJA, were approximately 100% in both mutants and wild-type (Col-0) seeds. This experiment was repeated five times with no less than 100 seeds per line and the differences between the lines were highly statistically significant (P < 0.001). Before any attempt to interpret these results is made, it is important to consider that Arabidopsis Gα mutants are less responsive to GA3 during germination than wild-type plants (Ullah et al., 2002), and impaired sensitivity to GA3 has also been reported for the rice Gα mutants (Ueguchi-Tanaka et al., 2000). The effect of GA3 is antagonistic to ABA during germination (Nambara et al., 1992; Ni and Bradford, 1993), making it important to eliminate the possible effect of endogenous GA3 in the observed germination rates. To highlight the role of MeJA, we treated all seeds with 10 μm paclobutrazol (PAC) to inhibit GA3 biosynthesis before sowing them on standard MS or MS plates supplemented with 50 μm MeJA alone (no ABA added). Preinhibition of GA3 synthesis had little effect in overall germination but now MeJA treatment had a clear inhibitory effect in germination of wild-type plants (P < 0.05; Fig. 5B). At the same time, gpa1-4 mutants were more severely affected than wild-type plants (P < 0.01), while agb1-2 showed no significant difference with or without MeJA treatment (Fig. 5B).
Finally, a typical response to MeJA exposure in Arabidopsis is a marked increase in the synthesis of anthocyanins (Feys et al., 1994). Treatment with 50 mm MeJA resulted in a noticeable increase in anthocyanin levels in wild-type and mutant plants but no significant differences were observed between the different genotypes (data not shown).
DISCUSSION
The involvement of G proteins in plant defense has repeatedly been suggested as a result of studies using pharmacological agents to modulate the activity of the α-subunit. Collectively, these experiments demonstrated that treatment of plants or plant tissue cultures with G-protein activators enhanced the plant resistance to certain pathogens, while treatment with G-protein inhibitors decreased the resistance (Legendre et al., 1992, 1993; Vera-Estrella et al., 1993, 1994; Xing et al., 1997; Perekhod et al., 1998; Rajasekhar et al., 1999; Roos et al., 1999; van der Luit et al., 2000; Kurosaki et al., 2001; Booker et al., 2004; Han and Yuan, 2004). The majority of these studies were specifically devoted to the oxidative burst and its components. However, there is at least one report speculating that G proteins may mediate elicitor signals in the jasmonate pathway leading to biosynthesis of the phytoalexin β-thujaplicin (Zhao and Sakai, 2003).
We used a genetic approach to study the involvement of the G proteins in plant defense against a variety of pathogens. Our results clearly show that Gβ-deficient mutants are more susceptible to infection with the necrotrophic pathogens A. brassicicola and F. oxysporum when compared to wild type (Col-0), while Gα-deficient mutants are less susceptible to the disease than wild type. According to the classical model for the G-protein mechanism of action, the lack of Gα subunit not only abolishes Gα-mediated signaling but also results in free Gβγ and therefore has the potential to increase the Gβγ signal output. In contrast, it is known in mammalian systems that Gβγ is required for the recruitment of Gα to the receptor and reactivation, and hence lack of Gβ prevents not only Gβγ but also Gα signaling (Clapham and Neer, 1993, 1997; Jones and Assmann, 2004). Therefore, the increased resistance observed in the Gα mutants could be accounted for by lack of the corresponding Gα-mediated signaling or by the constitutive activation of the Gβγ subunit. To rule out the former possibility, we studied the double-knockout mutant lacking both subunits. The behavior of the double mutant in response to F. oxysporum and A. brassicicola was indistinguishable from the single Gβ mutant showing the same degree of increased susceptibility to both pathogens when compared to wild-type and Gα mutants. This data strongly suggests that the Gβγ dimer, and not Gα, is the active subunit involved in the defense signaling pathway. Our results are consistent with a very recent report on the interaction of Arabidopsis gpa1-4 and agb1-1 mutants with the necrotrophic fungus P. cucumerina (Llorente et al., 2005).
JA-mediated defense signaling is an important component of plant resistance to necrotrophic fungi (Thomma et al., 1998; van Wees et al., 2003; Coego et al., 2005). In the Arabidopsis-A. brassicicola interaction, it was shown that ein2-1 and sid2 mutants with serious defects in ethylene and SA signaling, respectively, were resistant to the pathogen, while coronatine insensitive 1 mutant (impaired in JA sensitivity) was susceptible (van Wees et al., 2003). Moreover, the pad3 mutant, which is susceptible to A. brassicicola, has fully operational ethylene and JA signaling (Thomma et al., 1999b); however, pretreatment of the mutant with ethylene failed to confer protection against the pathogen, while MeJA reduced symptoms by 80% (Thomma et al., 1999b). These facts establish a crucial role of JA signaling in resistance to A. brassicicola. It was also shown that even the partially JA-insensitive mutant jar1-1 is susceptible to the necrotrophic pathogen Pythium irregulare (Tiryaki and Staswick, 2002). We have presented four independent lines of evidence indicating that G proteins are involved in JA signaling. (1) Lesions caused by A. brassicicola infection were more severe on Gβ mutants than wild-type leaves, while lesions on Gα mutants were smaller than those of wild-type plants. It has been clearly established that necrotic lesion development upon A. brassicicola infection is repressed by JA (Overmyer et al., 2003; Tuominen et al., 2004). (2) Plant defense-related genes are clearly affected in Gβ mutants after A. brassicicola attack, showing lower or delayed induction patterns. This was true for all genes studied but most importantly for PDF1.2, a plant defensin inducible by JA and ethylene (Penninckx et al., 1998). (3) Treatment of plants with exogenous MeJA showed that the induction of PDF1.2 was impaired in Gβ mutants. (4) Gβ mutants are partially insensitive to MeJA for a number of responses including MeJA-induced inhibition of root elongation, MeJA-induced inhibition of seed germination, and viability of plants in sublethal concentrations of MeJA. In addition, the behavior of the Gα mutants is coherent with the classic heterotrimeric mechanism of action for Gβγ-mediated signaling, showing the opposite effect to the Gβ mutants, namely, slightly increased resistance to fungi, increased expression of PDF1.2, and slightly higher sensitivity to MeJA than wild-type plants. All these observations strongly suggest that G proteins are actively involved in the regulation of JA signaling. The fact that only partial insensitivity to exogenous MeJA was observed suggests that the Gβγ subunit is not an integral component of the JA-signaling cascade but a positive regulator. We need to note that not all MeJA-mediated responses were affected on the G-protein mutants, as has been previously described for other JA-response mutants (Staswick et al., 1992; Schenk et al., 2000; Kachroo et al., 2003; Lorenzo et al., 2004); however, it is important to emphasize that all tested JA-mediated defense responses were affected in the mutants establishing a strong link between defense to necrotrophic pathogens, JA signaling, and G proteins.
The involvement of G proteins in the oxidative burst and subsequent disease resistance has been confirmed using Gα-null mutants in rice (Suharsono et al., 2002). In addition, studies of the response of gpa1-4 and agb1-2 Arabidopsis mutants to ozone stress also suggested the participation of the Gα subunit in the production of reactive oxygen species (Joo et al., 2005). The oxidative burst is considered to be an important factor restricting bacterial growth and in particular P. syringae. It was therefore surprising that our experiments with P. syringae did not reveal any differences in resistance to either virulent or avirulent strains of the bacteria between wild type and any of the G-protein mutants. In fact, our results seem to contradict the available pharmacological data as well as previous observations in Gα-deficient rice mutants. However, despite the frequent use of some pharmacological agents as G-protein activators or inhibitors, their specificity for activation of canonical G proteins in plants has been recently questioned (Fujisawa et al., 2001; Miles et al., 2004). It has been shown that mastoparan has pleiotropic effects on mitogen-activated protein kinases (Grant et al., 2000; Miles et al., 2004), affecting signaling to defense-related processes including the oxidative burst. Therefore, the use of pharmacological agents can elicit a stronger hypersensitive response (independent of G-protein activation), which in turn will result in a more pronounced effect on plant resistance. On the other hand, the rice Gα-deficient mutants showed decreased resistance to an avirulent race of Magnaporthe grisea, while infection with the virulent race did not reveal any differences between mutants and wild type (Suharsono et al., 2002). Interestingly, rice and Arabidopsis Gα-deficient mutants display very different phenotypes. As an example, the lack of the Gα subunit in rice causes severe dwarfism (Fujisawa et al., 1999), while in Arabidopsis a similar mutation produces rather the opposite effect, with mutants slightly larger than wild type (Ullah et al., 2003). These differences suggest that G proteins could have functionally diverged during evolution in monocots and dicots. Nevertheless, the observed apparent inconsistencies could be also explained by the choice of plant/pathogen combination and/or experimental conditions. Therefore, despite the similar resistance to P. syringae shown by the G-protein mutants in our study, we cannot rule out a role for these proteins in resistance to biotrophic pathogens.
An interesting observation from our seed germination assays is that the requirement for ABA to notice the effect of MeJA in Arabidopsis is due to the antagonistic role that ABA has with GA3 during germination. Inhibition of endogenous GA3 synthesis by pretreatment of the seeds with PAC resulted in an inhibition of germination by MeJA alone, without the need of additional ABA. We hypothesize that the decreased MeJA-mediated inhibition of germination observed in Arabidopsis is due to the effect of endogenous GA3.
CONCLUSION
In summary, our results clearly show that G proteins are involved in the plant defense against necrotrophic pathogens and that the Gβγ-functional subunit, but not Gα, mediates the response. We have also shown that G proteins, through the Gβγ-functional subunit, are implicated in JA signaling although are not an integral part of the signaling pathway. Although further proof is needed, we hypothesize that Gβγ acts as a direct or indirect enhancer of the JA pathway in plants and that the observed decrease in JA response in Gβ-deficient mutants could be the cause for the enhanced susceptibility to necrotrophic fungi.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) agb1-2, gpa1-3, and gpa1-4 mutants (SALK_061896, SALK_066823, and SALK_001846) were obtained from the Arabidopsis Biological Resource Center (ABRC; Ohio State University). These lines had been previously characterized as null mutants (Jones et al., 2003; Ullah et al., 2003). agb1-1 was obtained from the ABRC collection as an ethyl methanesulfonate point mutation, described by Lease et al. (2001). All lines have the same genetic background of Arabidopsis ecotype Col-0.
Pathogen Preparation and Inoculations
Fusarium oxysporum f. sp. conglutinans (BRIP 5176, Department of Primary Industries, Queensland, Australia) was grown on agar plates containing six to eight gamma-radiated sterilized carnation (Dianthus caryophyllus) leaves. Production of microconidia and root inoculations were performed as previously described (Campbell et al., 2003).
Alternaria brassicicola (isolate UQ4273) was grown on agar plates containing 10% (w/v) oats (Avena sativa; Uncle Tobys). Spores were prepared by irrigation of the plates with distilled water containing 0.01% (v/v) Tween 20 and draining through Miracloth. The spores were spun down and resuspended in sterile water to a final concentration of 106 spores/mL. Plants were inoculated with 5 μL of spore suspension placed onto the surface of individual leaves or by spraying plants with the spore suspension. Inoculated plants were kept in a growth chamber with controlled humidity. Mock inoculations were conducted using distilled water.
Pseudomonas syringae pv tomato strains DC3000 (virulent on wild-type Col-0) and JL1065 (avirulent on wild-type Col-0) were cultured in King's B medium (King et al., 1954) at 30°C. The antibiotics rifampicin and kanamycin were included in DC3000 cultures at the concentrations of 50 μg/mL each, while strain JL1065 was grown in the presence of 50 μg/mL rifampicin only. Leaves of 3- to 4-week-old Arabidopsis plants were inoculated using syringe. Control infiltrations were conducted with sterile 10 mm MgCl2. Measurements of in planta bacterial growth were conducted as previously described (Mohr and Cahill, 2003).
Hormone Treatments
Plants were surface sprayed with 50 μm MeJA (Sigma) or 5 μm SA (Sigma) dissolved in sterile water until or exposed to 200 ppm ethylene. Control plants were treated the same way without the addition of signaling molecules.
Plate Assays
Standard plates were made with 1× MS basal salts (PhytoTechnology Laboratories), 1% Suc, and 0.8% agar unless otherwise stated. Stock solutions of MeJA and ABA were added to autoclaved medium cooled down to approximately 55°C to designated concentrations. PAC was added to sterilized seeds from a 20 μm or 200 μm ethanolic stock solution to a final concentration of 10 μm or 100 μm. After 48-h incubation at 4°C seeds were washed with ample amounts of sterile water. Germination was determined as an obvious protrusion of the radicle. For root assays, seedlings were grown in plates vertically for 14 d, then photographed and measured.
Isolation of RNA and Northern-Blot Analysis
Total RNA for northern analysis was extracted with the SVtotal RNA isolation kit (Promega). Probes were labeled using a Rediprime II P32 radiolabeling kit (Amersham). Membranes were hybridized overnight in Church buffer (Church and Gilbert, 1984) and exposed to Phosphorimager plates for analysis (Molecular Dynamics).
Acknowledgments
Wild-type Col-0 and G-protein mutants agb1-1, agb1-2, gpa1-3, gpa1-4, and the double mutant gpa1-4/agb1-2 were kindly provided by the ABRC and Dr. Alan Jones (University of North Carolina). We wish to thank Dr. Kemal Kazan (University of Queensland) for critical reading of the manuscript.
This work was supported by the Australian Research Council (grant no. DP0344924).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: José Ramón Botella (j.botella@uq.edu.au).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.069625.
References
- Agrios GN (2005) Plant Pathology, Ed 5. Elsevier Academic Press, New York
- Ashikari M, Wu JZ, Yano M, Sasaki T, Yoshimura A (1999) Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc Natl Acad Sci USA 96: 10284–10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffa R, Szell M, Meuwly P, Pay A, Vogeli-Lange R, Metraux J-P, Neuhaus G, Meins F Jr, Nagy F (1995) Cholera toxin elevates pathogen resistance and induces pathogenesis-related gene expression in tobacco. EMBO J 14: 5753–5761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesgen C, Weiler EW (1999) Structure and regulation of OPR1 and OPR2, two closely related genes encoding 12-oxophytodienoic acid-10,11-reductases from Arabidopsis thaliana. Planta 208: 155–165 [DOI] [PubMed] [Google Scholar]
- Booker FL, Burkey KO, Overmyer K, Jones AM (2004) Differential responses of G-protein Arabidopsis thaliana mutants to ozone. New Phytol 162: 633–641 [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Schenk PM, Kazan K, Penninckx IAMA, Anderson JP, Maclean DJ, Cammue BPA, Ebert PR, Manners JM (2003) Pathogen-responsive expression of a putative ATP-binding cassette transporter gene conferring resistance to the diterpenoid sclareol is regulated by multiple defense signaling pathways in Arabidopsis. Plant Physiol 133: 1272–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci USA 81: 1991–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D, Neer E (1993) New roles for G-protein βγ-dimers in transmembrane signalling. Nature 365: 403–406 [DOI] [PubMed] [Google Scholar]
- Clapham D, Neer E (1997) G protein subunits. Annu Rev Pharmacol Toxicol 37: 167–203 [DOI] [PubMed] [Google Scholar]
- Coego A, Ramirez V, Gil MJ, Flors V, Mauch-Mani B, Vera P (2005) An Arabidopsis homeodomain transcription factor, OVEREXPRESSOR OF CATIONIC PEROXIDASE 3, mediates resistance to infection by necrotrophic pathogens. Plant Cell 17: 2123–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn KL, Tewari JP, Dahiya JS (1988) Resistance to Alternaria brassicae and phytoalexin elicitation in rapeseed and other crucifers. Plant Sci 56: 21–25 [Google Scholar]
- Dong XN, Mindrinos M, Davis KR, Ausubel FM (1991) Induction of Arabidopsis defense genes by virulent and avirulent Pseudomonas syringae strains and by a cloned avirulence gene. Plant Cell 3: 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan LM, Zhao ZX, Assmann SM (2004) Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7: 537–546 [DOI] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG (1994) Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 6: 751–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa Y, Kato H, Iwasaki Y (2001) Structure and function of heterotrimeric G proteins in plants. Plant Cell Physiol 42: 789–794 [DOI] [PubMed] [Google Scholar]
- Fujisawa Y, Kato T, Ohki S, Ishikawa A, Kitano H, Sasaki T, Asahi T, Iwasaki Y (1999) Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc Natl Acad Sci USA 96: 7575–7580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelli A, Higgins VJ, Blumwald E (1997) Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiol 113: 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JJ, Yun B-W, Loake GJ (2000) Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J 24: 569–582 [DOI] [PubMed] [Google Scholar]
- Han RB, Yuan YJ (2004) Oxidative burst in suspension culture of Taxus cuspidata induced by a laminar shear stress in short-term. Biotechnol Prog 20: 507–513 [DOI] [PubMed] [Google Scholar]
- Jones AM, Assmann SM (2004) Plants: the latest model system for G-protein research. EMBO Rep 5: 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Ecker JR, Chen J-G (2003) A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis. Plant Physiol 131: 1623–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo J, Wang S, Chen J, Jones A, Fedoroff N (2005) Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell 17: 957–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A, Lapchyk L, Fukushige H, Hildebrand D, Klessig D, Kachroo P (2003) Plastidial fatty acid signaling modulates salicylic acid- and jasmonic acid-mediated defense pathways in the Arabidopsis ssi2 mutant. Plant Cell 15: 2952–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan IA, Hammerschmidt R (2002) Arabidopsis ecotype variability in camalexin production and reaction to infection by Alternaria brassicicola. J Chem Ecol 28: 2121–2140 [DOI] [PubMed] [Google Scholar]
- King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44: 301–307 [PubMed] [Google Scholar]
- Komatsu S, Yang G, Hayashi N, Kaku H, Umemura K, Iwasaki Y (2004) Alterations by a defect in a rice G protein α subunit in probenazole and pathogen-induced responses. Plant Cell Environ 27: 947–957 [Google Scholar]
- Kurosaki F, Yamashita A, Arisawa M (2001) Involvement of GTP-binding protein in the induction of phytoalexin biosynthesis in cultured carrot cells. Plant Sci 161: 273–278 [DOI] [PubMed] [Google Scholar]
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC (2001) A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell 13: 2631–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre L, Heinstein P, Low P (1992) Evidence for participation of GTP-binding proteins in elicitation of the rapid oxidative burst in cultured soybean cells. J Biol Chem 267: 20140–20147 [PubMed] [Google Scholar]
- Legendre L, Rueter S, Heinstein PF, Low PS (1993) Characterization of the oligogalacturonide-induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiol 102: 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente F, Alonso-Blanco C, Sanchez-Rodriguez C, Jorda L, Molina A (2005) ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J 43: 165–180 [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Chico JM, Sanchez-Serrano JJ, Solano R (2004) JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H (1994) GTP-binding proteins in plants: new members of an old family. Plant Mol Biol 26: 1611–1636 [DOI] [PubMed] [Google Scholar]
- Marrs KA (1996) The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 127–158 [DOI] [PubMed] [Google Scholar]
- Mason MG, Botella JR (2000) Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc Natl Acad Sci USA 97: 14784–14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR (2001) Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim Biophys Acta 1520: 147–153 [DOI] [PubMed] [Google Scholar]
- Mauchmani B, Slusarenko AJ (1994) Systemic acquired resistance in Arabidopsis thaliana induced by a predisposing infection with a pathogenic isolate of Fusarium oxysporum. Mol Plant Microbe Interact 7: 378–383 [Google Scholar]
- Miles GP, Samuel MA, Jones AM, Ellis BE (2004) Mastoparan rapidly activates plant MAP kinase signaling independent of heterotrimeric G proteins. Plant Physiol 134: 1332–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PG, Cahill DM (2003) Abscisic acid influences the susceptibility of Arabidopsis thaliana to Pseudomonas syringae pv. tomato and Peronospora parasitica. Funct Plant Biol 30: 461–469 [DOI] [PubMed] [Google Scholar]
- Nambara E, Naito S, McCourt P (1992) A mutant of Arabidopsis which is defective in seed development and storage protein accumulation is a new ABI3 allele. Plant J 2: 435–441 [Google Scholar]
- Ni BR, Bradford KJ (1993) Germination and dormancy of abscisic acid-deficient and gibberellin-deficient mutant tomato (Lycopersicon esculentum) seeds: sensitivity of germination to abscisic-acid, gibberellin, and water potential. Plant Physiol 101: 607–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Brosche M, Kangasjarvi J (2003) Reactive oxygen species and hormonal control of cell death. Trends Plant Sci 8: 335–342 [DOI] [PubMed] [Google Scholar]
- Penninckx I, Eggermont K, Terras F, Thomma B, Samblanx G, Buchala A, Metraux JP, Manners JM, Broekaert WF (1996) Pathogen induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 8: 2309–2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx I, Thomma B, Buchala A, Metraux JP, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perekhod EA, Chalenko GI, Il'inskaya LI, Vasyukova NI, Gerasimova NG, Babakov AV, Usov AI, Mel'nikova TM, Ozeretskovskaya OL (1998) Modulation of potato resistance by means of xyloglucan fragments. Appl Biochem Microbiol 34: 91–96 [Google Scholar]
- Preston CA, Betts H, Baldwin IT (2002) Methyl jasmonate as an allelopathic agent: sagebrush inhibits germination of a neighboring tobacco, Nicotiana attenuata. J Chem Ecol 28: 2343–2369 [DOI] [PubMed] [Google Scholar]
- Rajasekhar VK, Lamb C, Dixon RA (1999) Early events in the signal pathway for the oxidative burst in soybean cells exposed to avirulent Pseudomonas syringae pv glycinea. Plant Physiol 120: 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S (2000) Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol 124: 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos W, Dordschbal B, Steighardt J, Hieke M, Weiss D, Saalbach G (1999) A redox-dependent, G-protein-coupled phospholipase A of the plasma membrane is involved in the elicitation of alkaloid biosynthesis in Eschscholtzia californica. Biochim Biophys Acta 1448: 390–402 [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Manners JM, Anderson JP, Simpson RS, Wilson IW, Somerville SC, Maclean DJ (2003) Systemic gene expression in Arabidopsis during an incompatible interaction with Alternaria brassicicola. Plant Physiol 132: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97: 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P, Su W, Howell S (1992) Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc Natl Acad Sci USA 89: 6837–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K (2002) The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 99: 13307–13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Broekaert WF, Cammue BPA (2000) Disease development of several fungi on Arabidopsis can be reduced by treatment with methyl jasmonate. Plant Physiol Biochem 38: 421–427 [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFMJ, Broekaert WF (1999. a) Requirement of functional ethylene-insensitive 2 gene for efficient resistance of Arabidopsis to infection by Botrytis cinerea. Plant Physiol 121: 1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Neilssen I, Eggermont K, Brokeart WF (1999. b) Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J 19: 163–171 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Tierens KFM, Penninckx IAMA, Mauch-Mani B, Broekaert WF, Cammue BPA (2001) Different micro-organisms differentially induce Arabidopsis disease response pathways. Plant Physiol Biochem 39: 673–680 [Google Scholar]
- Tiryaki I, Staswick PE (2002) An Arabidopsis mutant defective in jasmonate response is allelic to the auxin-signaling mutant axr1. Plant Physiol 130: 887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Overmyer K, Keinanen M, Kollist H, Kangasjarvi J (2004) Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. Plant J 39: 59–69 [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Fujisawa Y, Kobayashi M, Ashikari M, Iwasaki Y, Kitano H, Matsuoka M (2000) Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc Natl Acad Sci USA 97: 11638–11643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen J-G, Temple B, Boyes DC, Alonso JM, Davis KR, Ecker JR, Jones AM (2003) The β-subunit of the Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell 15: 393–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen J-G, Wang S, Jones AM (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen J-G, Young JC, Im K-H, Sussman MR, Jones AM (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292: 2066–2069 [DOI] [PubMed] [Google Scholar]
- van der Luit AH, Piatti T, van Doorn A, Musgrave A, Felix G, Boller T, Munnik T (2000) Elicitation of suspension-cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol 123: 1507–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees SCM, Chang HS, Zhu T, Glazebrook J (2003) Characterization of the early response of Arabidopsis to Alternaria brassicicola infection using expression profiling. Plant Physiol 132: 606–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla BJ, Higgins VJ, Blumwald E (1993) G-protein mediated plant defense response to a cultivar-specific fungal pathogen. Plant Physiol 102: 2112231795 [Google Scholar]
- Vera-Estrella R, Higgins VJ, Blumwald E (1994) Plant defense response to fungal pathogens: G-protein-mediated changes in host plasma-membrane redox reactions. Plant Physiol 106: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P, Shockey J, Levesque CA, Cook RJ, Browse J (1998) A role for jasmonate in pathogen defense of Arabidopsis. Proc Natl Acad Sci USA 95: 7209–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-Q, Ullah H, Jones AM, Assmann SM (2001) G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292: 2070–2072 [DOI] [PubMed] [Google Scholar]
- Weiss C, Garnaat C, Mukai K, Hu Y, Ma H (1994) Isolation of cDNAs encoding guanine nucleotide-binding protein β-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc Natl Acad Sci USA 91: 9554–9558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen RW, Vanrooijen GJH, Pearce DW, Pharis RP, Holbrook LA, Moloney MM (1991) Effects of jasmonic acid on embryo-specific processes in Brassica and Linum oilseeds. Plant Physiol 95: 399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E (1997) Identification of G proteins mediating fungal elicitor-induced dephosphorylation of host plasma membrane H+-ATPase. J Exp Bot 48: 229–237 [Google Scholar]
- Zhao J, Sakai K (2003) Multiple signalling pathways mediate fungal elicitor-induced β-thujaplicin biosynthesis in Cupressus lusitanica cell cultures. J Exp Bot 54: 647–656 [DOI] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Glazebrook J (1999) Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell 11: 2419–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]