Abstract
Most seeds are anhydrobiotes, relying on an array of protective and repair mechanisms, and seed mitochondria have previously been shown to harbor stress proteins probably involved in desiccation tolerance. Since temperature stress is a major issue for germinating seeds, the temperature response of pea (Pisum sativum) seed mitochondria was examined in comparison with that of mitochondria from etiolated epicotyl, a desiccation-sensitive tissue. The functional analysis illustrated the remarkable temperature tolerance of seed mitochondria in response to both cold and heat stress. The mitochondria maintained a well-coupled respiration between −3.5°C and 40°C, while epicotyl mitochondria were not efficient below 0°C and collapsed above 30°C. Both mitochondria exhibited a similar Arrhenius break temperature at 7°C, although they differed in phospholipid composition. Seed mitochondria had a lower phosphatidylethanolamine-to-phosphatidylcholine ratio, fewer unsaturated fatty acids, and appeared less susceptible to lipid peroxidation. They also accumulated large amounts of heat shock protein HSP22 and late-embryogenesis abundant protein PsLEAm. The combination of membrane composition and stress protein accumulation required for desiccation tolerance is expected to lead to an unusually wide temperature tolerance, contributing to the fitness of germinating seeds in adverse conditions. The unique oxidation of external NADH at low temperatures found with several types of mitochondria may play a central role in maintaining energy homeostasis during cold shock, a situation often encountered by sessile and ectothermic higher plants.
Many organisms need to cope with extreme temperatures, but few are adapted to live and reproduce in such conditions. While extremophilic microorganisms can metabolically adapt, more complex organisms avoid temperature stress by controlling body temperature or by moving to more favorable habitats. As land plants are ectothermic and unable to move, they cannot escape dramatic changes in temperature. Most live in environments where frequent temperature changes of 10°C to 20°C are common, and some, such as alpine plants, may experience fluctuations of more than 40°C in a single day. While much work has been carried out on the acclimation of plants to either low or high temperature, little is known about the mechanisms allowing them to cope with sudden temperature fluctuations that may exist for extended periods. In analyzing this situation, we obtained evidence from seeds that mitochondria play a central role in allowing plants to adapt to extreme temperatures.
In the life cycle of higher plants, seeds must complete the crucial task of protecting the embryo and driving it toward the establishment of a new generation. The majority of higher plant seeds are desiccation tolerant, a complex trait that has contributed to the evolutionary success of angiosperms. Desiccation-tolerant seeds are in fact anhydrobiotes and certainly represent the most stress-tolerant stage of plants. They are endowed with an impressive longevity that ranges from years to centuries, depending on the species (Walters et al., 2005), and confers a clear, competitive advantage in the environment. It can be expected that mechanisms involved in the extreme resistance of seeds to desiccation might provide the germinating seed with cross-tolerance to other abiotic stresses, such as temperature. This type of stress has great significance because it represents a major limitation for agriculture. Considerable effort has been devoted to understanding the molecular basis of resistance to heat, chilling, and freezing, and it has been revealed that plants react to temperature variations by alterations in metabolic rates, protein turnover, osmolytes, membrane function, and gene expression (Smallwood and Bowles, 2002; Sung et al., 2003). All these processes require energy and thus depend on the competence and stability of mitochondria, which were, for instance, clearly involved in the recovery of maize (Zea mays) seedlings from chilling (Prasad et al., 1994). Low and high temperatures have pronounced and complex effects on mitochondrial properties in terms of both their function and their biogenesis. Among the effects of cold treatment of plant tissues on mitochondria, the most notable are reversible uncoupling (Popov et al., 2002), decrease in protein import (Taylor et al., 2003), and accumulation of stress-related proteins, such as peroxidase (Prasad et al., 1994), catalase (Prasad et al., 1995), dehydrin-like protein (Borovskii et al., 2000), and uncoupling protein (UCP; Kolesnichenko et al., 2000). In pea (Pisum sativum) seedlings, heat shock has been shown to induce rapid synthesis of heat shock protein 22 (HSP22), a small HSP that has been localized in mitochondria (Lenne and Douce, 1994; Lenne et al., 1995). In their pioneering work, Lyons and Raison (1970) found that mitochondria from chilling-sensitive plant tissues exhibited dramatic increases in the activation energy of succinate oxidation at critical temperatures between 9°C and 12°C. Such an effect was not found with mitochondria from chilling-tolerant species, suggesting the occurrence of mitochondrial adaptation to cold. The critical temperature of activation energy change was related to lipid phase transition, suggesting a correlation between the physical state of the membranes and the biochemical activities (Raison et al., 1971).
A comparative analysis of mitochondria from two maize genotypes selected for their tolerance to cold germination revealed a higher percentage of 18-carbon unsaturated fatty acids (FAs), a higher fluidity, and a higher activity of cytochrome c oxidase for mitochondrial inner membranes of the cold-tolerant population (De Santis et al., 1999). Such results correspond well with the idea of modification of lipid composition to maintain membrane fluidity in response to temperature change, the so-called homeoviscous adaptation (Sinensky, 1974). A relationship between growth temperature changes and mitochondrial membrane properties was also found in the case of soybean (Glycine max) seedlings, but the functional adaptive trend was only apparent at high temperatures in agreement with the cold sensitivity of the species (Davy de Virville et al., 2002). In the short term (i.e. without acclimation), the response of mitochondrial respiration is likely to be controlled by adenylates and substrate supplies at moderate temperatures, and restricted by enzyme and membrane function at extreme temperatures (Atkin et al., 2000; Atkin and Tjoelker, 2003). To operate in stressful conditions, plant mitochondria might have evolved distinctive features designed to increase their metabolic flexibility and stress tolerance, which is clearly illustrated by both the complexity of the mitochondrial proteome and by its dynamic response to environmental stress (Heazlewood et al., 2004; Millar et al., 2005; Taylor et al., 2005). In addition to the classical complexes, all plant mitochondria possess alternative enzymes that confer on them the capacity to oxidize external NAD(P)H or internal NAD(P)H (NDin) independently of complex I and to transfer electrons directly from reduced ubiquinone to oxygen via an alternative oxidase (AOX; Douce and Neuburger, 1989). Although the general physiological roles of these alternative pathways have not been established, they are expected to provide additional flexibility to mitochondrial metabolism in situations of stress (Vanlerberghe and McIntosh, 1997; Rasmusson et al., 2004). In germinating seeds, mitochondria provide most of the cellular ATP because germination is a heterotrophic process that is powered by the utilization of energy stored during seed development. It has been shown that mitochondria in dry seeds rapidly become active during imbibition, relying on external NADH and succinate as substrates (Logan et al., 2001; Benamar et al., 2003). To remain functional throughout desiccation, it is therefore expected that seed mitochondria benefit from protective or repair mechanisms at the protein and membrane levels. Stress proteins such as HSP22 and a late-embryogenesis abundant (LEA) protein (PsLEAm) accumulate to high levels in the matrix of pea seed mitochondria during seed development (Bardel et al., 2002; Grelet et al., 2005). To gain more insight into the temperature tolerance of seed mitochondria, the organelles from desiccation-tolerant seeds and from desiccation-sensitive pea seedling epicotyls were functionally and biochemically characterized under a wide range of temperatures. The results highlighted the remarkable tolerance of seed mitochondria to temperature stress and indicate a major role of external NADH oxidation in the cold.
RESULTS
Temperature Response of Oxidative Phosphorylation in Seed and Epicotyl Mitochondria
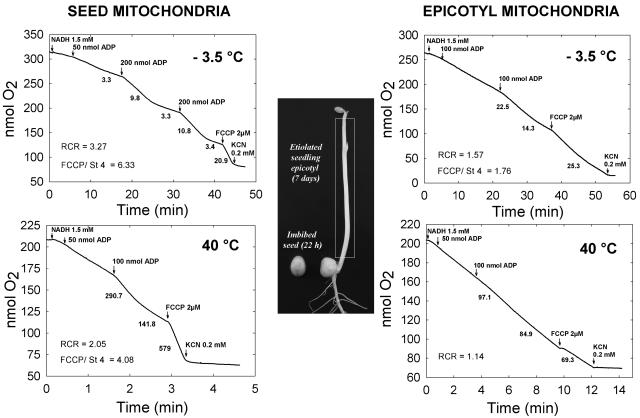
The plant material used for mitochondrial isolation was either 22-h imbibed seeds or 7-d-old etiolated epicotyls (Fig. 1). Seeds had a water content of 75% (fresh-weight basis) and were still desiccation tolerant (Benamar et al., 2003), while the etiolated epicotyls, which had a high water content (94%, fresh-weight basis), were obviously desiccation sensitive. Both types of mitochondria were purified on Percoll gradients, yielding intact and functional organelles. According to marker enzymes and pigment analysis, the contamination by cytosol, plastids, or peroxisomes was negligible (data not shown). Nevertheless, seed mitochondria were slightly contaminated by protein bodies as was revealed by previous proteomic analysis (Bardel et al., 2002). The oxidative properties of isolated mitochondria were tested under a wide range of physiological temperatures from 40°C down to below the freezing point. NADH was selected as a substrate because it was well oxidized by both types of mitochondria, even at very low temperatures (Table I). As can be deduced from oxygen consumption rates and the respiratory control ratio (RCR), other substrates, such as succinate or malate-Glu, could not sustain an efficient oxidative phosphorylation at low temperatures, highlighting the performance of external NADH oxidation in the cold (Table I). With mitochondria isolated from the hypocotyls of Mung bean (Phaseolus aureus), a chilling-sensitive species, NADH also appeared as the only substrate able to sustain oxidative phosphorylation at low temperatures (data not shown). NADH was thus selected as the substrate for comparing the temperature responses of pea seed and epicotyl mitochondria between −3.5°C and 40°C. Both types of mitochondria exhibited considerable differences in their functioning at extreme temperatures (Fig. 1). At a temperature as low as −3.5°C, just above the freezing point of the assay buffer, seed mitochondria demonstrated an excellent coupling of oxidative phosphorylation with a RCR over 3 and a strong stimulation of state 4 rate by the uncoupler p-trifluoromethoxycarbonyl-cyanide (FCCP). Under the same conditions, epicotyl mitochondria showed a higher oxidation rate, but with a much lower RCR and FCCP stimulation (Fig. 1). At 40°C, while seed mitochondria oxidized NADH at a high rate with strong ADP and FCCP-dependent stimulations, the epicotyl organelles exhibited a markedly reduced oxidation rate and a loss of coupling. On the whole, these observations indicate that seed mitochondria perform much better than their epicotyl counterparts in terms of oxidative phosphorylation at extreme temperatures.
Figure 1.
NADH oxidation by pea seed and epicotyl mitochondria at −3.5°C and 40°C. The photograph illustrates the pea seed and seedling material grown at 20°C and used for mitochondria isolation, the white rectangle indicating the epicotyl. Representative oxygraphic experiments performed at −3.5°C (top) or 40°C (bottom) are shown for seed mitochondria on the left and epicotyl mitochondria on the right. The arrows indicate addition of various compounds and numbers under the traces refer to the rates of oxygen consumption in nanomoles O2 per minute per milligram protein. The RCR (state 3/state 4 rates) and the FCCP uncoupler stimulation ratio are shown when appropriate.
Table I.
Substrate oxidation by pea seed and epicotyl mitochondria at low temperatures
Seed or epicotyl mitochondria respiration was measured with several substrates at low temperatures. Data correspond to an average of one to three repeats in a single experiment. State 3 oxidation rates are indicated in nanomoles O2 per minute per milligram protein, and the RCR is indicated in brackets. nd, Not detected.
| Temperature
|
State 3 Rate (RCR)
|
|||||
|---|---|---|---|---|---|---|
| NADH
|
Succinate
|
Malate-Glu
|
||||
| Seed | Epicotyl | Seed | Epicotyl | Seed | Epicotyl | |
| 5°C | 35.6 (3.2) | 105.6 (3.3) | 33.2 (3.5) | 69.3 (2.7) | 9.6 (2.4) | 29.6 (2.3) |
| 0°C | 18.8 (3.1) | 40.0 (2.1) | 8.4 (2.3) | 5.1 (1.0) | nd | nd |
| −3.5°C | 10.8 (3.3) | 22.5 (1.8) | 2.4 (1.0) | nd | – | – |
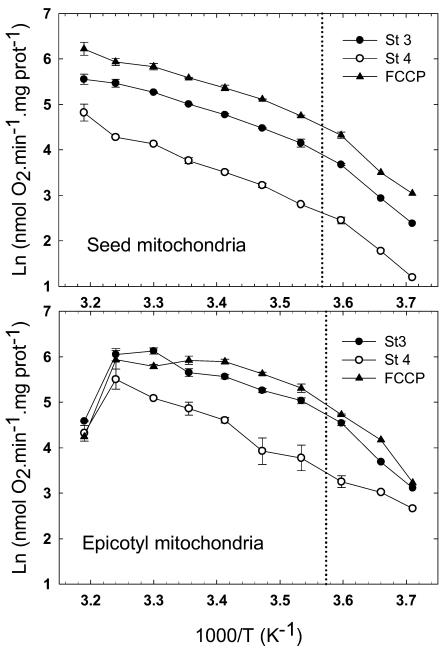
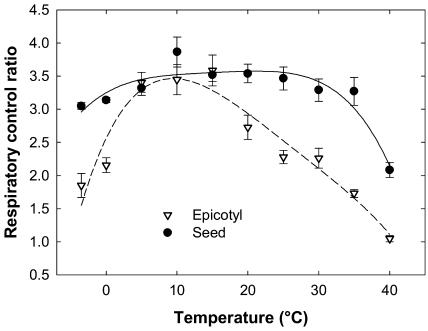
The response of both types of mitochondria was analyzed over the full temperature range between the extremes of −3.5°C and 40°C. To obtain a thermodynamic visualization of the effect of temperature on mitochondrial functioning, the oxygraphic data were plotted on Arrhenius graphs (Fig. 2). The Arrhenius profiles revealed striking differences between both types of mitochondria, confirming the above observations at extreme temperatures. In the case of seed mitochondria, the rates of state 4, state 3, and FCCP-uncoupled respiration steadily increased from −3.5°C to 40°C. The three curves are parallel, indicating a good coupling of mitochondria at all temperatures. An Arrhenius break temperature (ABT), shown in Figure 2, was estimated at 7.3°C for state 3 respiration by intersecting two linear regressions fitted to the curve (data not shown). The regression slopes were used to calculate the energy of activation of state 3 NADH oxidation, which increased from 39 kJ mol−1 in the 30°C to 10°C range to 92 kJ mol−1 under the ABT (7.3°C), at temperatures from 5°C to −3.5°C. Epicotyl mitochondria were significantly affected in warm conditions, their state 4, state 3, and FCCP-uncoupled respiration rates collapsing above 30°C with an evident loss of coupling (Fig. 2). On the other end of the scale, although there was apparently no severe drop in rates, uncoupled and state 3 rates of epicotyl mitochondria decreased more than their state 4 rate, which resulted in a decline in coupling. The estimation of ABT for state 3 rates indicated a value of 6.9°C, and energy of activation increased from 37 kJ mol−1 above to 105 kJ mol−1 below ABT. To support the results of the Arrhenius graph analysis, which seemed to indicate a wide tolerance of seed mitochondria to critical temperatures, the RCR was plotted as a function of temperature for both types of mitochondria (Fig. 3). The graph clearly illustrated the remarkable tolerance of seed mitochondria, which remained highly coupled (RCRs over 3) from −3.5°C up to 35°C, the RCR decreasing to 2.2 at 40°C. The epicotyl mitochondria exhibited a much lower tolerance to temperature fluctuations with good coupling only in the 5°C to 20°C range, the RCR dropping below 0°C or above 25°C.
Figure 2.
Effect of temperature on NADH oxidation by pea seed and epicotyl mitochondria. Shown are Arrhenius plots for state 3 (black circles), state 4 (white circles), and uncoupled (black triangles) respiration of pea seed and epicotyl mitochondria within the temperature range of 40°C to −3.5°C with NADH as a substrate. The data correspond to the mean of three repeats with se indicated. The vertical dotted line represents the ABT that was graphically determined using the intersect of linear regressions.
Figure 3.
Temperature dependence of RCR for NADH oxidation by seed and epicotyl mitochondria. The RCR for seed (black circles) and epicotyl (white triangles) mitochondria was averaged from two to five replicates and ses are indicated. Polynomial regression for seed (solid line) and epicotyl (dashed line) mitochondria are shown as a visual aid.
Lipid Composition and Peroxidation Susceptibility of Seed and Epicotyl Mitochondria
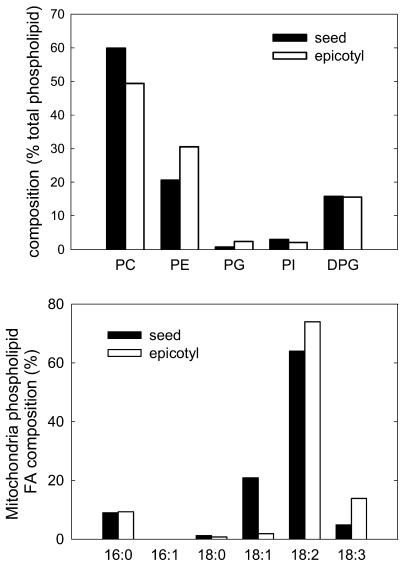
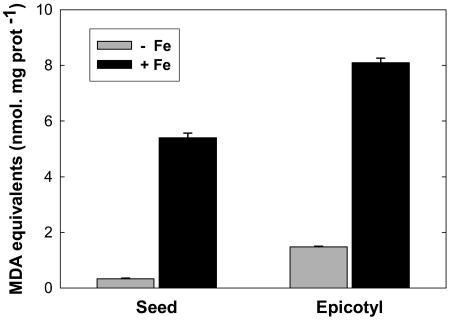
Considering the well-known interaction between temperature and membrane lipid composition (Hazel, 1995), as illustrated in the case of plant mitochondria during temperature acclimation (De Santis et al., 1999; Davy de Virville et al., 2002), the lipid and FA composition of the seed and epicotyl mitochondria were determined. The polar lipid composition revealed a classic mitochondrial profile dominated by phosphatidylcholine (PC), phosphatidylethanolamine (PE), and diphosphatidylglycerol (DPG), while phosphatidylglycerol (PG) and phosphatidylinositol (PI) were less abundant (Fig. 4). A difference was found in the PE-to-PC ratio, which appeared lower in seed mitochondria (0.34) than in epicotyl mitochondria (0.62), both of these compounds representing 80% of the polar lipids in both organelles (Fig. 4). The FA analysis revealed that epicotyl mitochondria were enriched in polyunsaturated species C18:2 and C18:3 (Fig. 4). Accordingly, the double-bond index (DBI = [N × mol % FA]/100, where N is the number of double bonds in each FA molecule) was much higher for epicotyl (1.916) than for seed mitochondria (1.636), suggesting a lower fluidity of the latter. Since a higher proportion of polyunsaturated FAs (PUFAs) may lead to a higher susceptibility of membrane lipids to oxidative stress, the amount of lipid peroxidation products including 4-hydroxyalkenals (HAE) and malondialdehyde (MDA) was determined. Although it can be presumed that endogenous lipid peroxidation products were lost during mitochondrial isolation, purified epicotyl mitochondria contained around 3 times more MDA equivalent than seed mitochondria (Fig. 5). When the organelles were exposed to severe oxidative conditions by incubation with 500 μm FeSO4, the peroxidation products accumulated to a higher extent in the case of epicotyl mitochondria (Fig. 5).
Figure 4.
Phospholipid and FA composition of mitochondria isolated from germinating seeds and seedlings of pea. Phospholipids (top) and their FA composition (bottom) are indicated as the percentage of weight. The phospholipids are PC, PE, PG, PI, and DPG. The FA labels indicate the length of the acid chain followed by the number of double bonds.
Figure 5.
Lipid peroxidation in seed and epicotyl mitochondria. The total amount of MDA and HAE were measured in seed and epicotyl mitochondria either without (−Fe, gray bars) or with (+Fe, black bars) oxidative stress induced by incubation of organelles with 500 μm FeSO4 for 1 h at 25°C. The error bars correspond to se.
Accumulation of Two Stress-Related Proteins in Seed Mitochondria
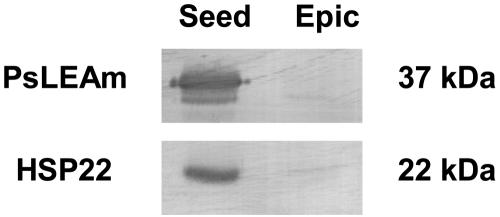
The accumulation of HSP22 and of a LEA protein (PsLEAm) in pea seed mitochondria have been reported recently (Bardel et al., 2002; Grelet et al., 2005). Specific antibodies were used to compare the amount of HSP22 and PsLEAm in both types of mitochondria by western blotting. The results confirmed the strong accumulation of both proteins in seed mitochondria and indicated that only traces of the stress protein could be detected in epicotyl mitochondria (Fig. 6). These may correspond to remnants of mitochondria from seed axes in the elongated epicotyls. It is therefore clear that a marked difference between seed and epicotyl mitochondria lies in their amounts of HSP22 and PsLEAm, which are constitutively expressed during seed development.
Figure 6.
Western-blot analysis of PsLEAm and HSP22 proteins in pea seed and epicotyl mitochondria. Mitochondrial proteins (30 μg) were separated by SDS-PAGE and immunoblotting analysis was performed with polyclonal antibodies against PsLEAm and HSP22. Equivalent loading and transfer of proteins to nitrocellulose were verified by Ponceau red staining.
DISCUSSION
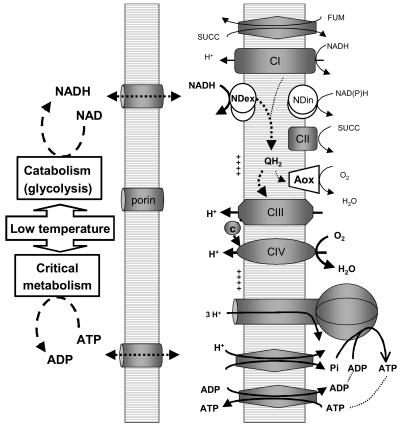
To characterize the temperature tolerance of mitochondria isolated from desiccation-tolerant seeds, they were compared with mitochondria isolated from desiccation-sensitive etiolated epicotyls. When investigating the function of those mitochondria from non-cold-acclimated plants in the lower range of physiological temperatures, we found that exogenous NADH was able to fuel respiration when other substrates failed, and thus to power oxidative phosphorylation at surprisingly low temperatures around 0°C and even below in the case of seeds. The rapid oxidation of exogenous NADH, characteristic of plant mitochondria, is driven by non-proton-pumping rotenone-insensitive NADH dehydrogenases (NDex) probably located on the outer side of the mitochondrial inner membrane (Rasmusson et al., 2004). The physiological role of exogenous NADH oxidation by plant mitochondria is not fully understood, although a number of hypotheses point toward stress tolerance in association with AOX (Rasmusson et al., 2004). According to our observations, we propose that one major physiological role for exogenous NADH oxidation by plant mitochondria could be to enable oxidative phosphorylation at low temperatures to maintain ATP levels while allowing primary and critical metabolism to proceed (Fig. 7). Such a coupling would allow cells to manage low-temperature crises by making invaluable bioenergetic use of any cytosolic NADH produced by temperature-depressed metabolism (e.g. glycolysis).
Figure 7.
Diagram of external NADH oxidation by plant mitochondria under cold conditions. The diagram shows the mitochondrial oxidative phosphorylation machinery, the specific plant units being shown as white shapes (NDex and NDin refer, respectively, to the external and internal NAD(P)H dehydrogenases, and AOX to the alternative oxidase). In cold crisis conditions, the oxidation of exogenous NADH is capable of sustaining ATP synthesis. In seeds, the system was shown to operate at a temperature as low as −3.5°C.
Pea seed mitochondria were capable of a well-coupled oxidative phosphorylation at very low temperatures, the lowest record being obtained at −3.5°C. It was not possible to further decrease temperature because of medium freezing and the limitations of the oxygraphic technique.
The proper functioning of these mitochondria at −3.5°C (to our knowledge the lowest recorded for any organism) is intriguing. The observed coupled oxidation of exogenous NADH provides evidence that metabolite transporters (adenylate nucleotides, phosphate), electron and proton transfer systems (NDex, ubiquinones, complex III, cytochrome c, complex IV), and the sophisticated ATP synthase all function correctly within a lipid bilayer that still remains fluid at such a low temperature (see Fig. 7). Under such adverse conditions, the system does not require the complex mitochondrial matrix enzymes of carbon metabolism and relies instead on the essential elements for oxidative phosphorylation that are directly fueled by NDex alone. It is worth noting that seed mitochondria that have the ability to withstand extreme loads during desiccation and imbibition were found to rely mainly upon exogenous NADH and succinate as substrates (Logan et al., 2001; Benamar et al., 2003). Thus, the simple exogenous NADH oxidative system seems appropriate for coping with different stress conditions such as those found in the context of seed development and germination or during cold stress. Taken together, our results suggest that the ubiquitous occurrence of NDex in plant mitochondria could provide most ectothermic plant tissues with an inherent ability to manage temporary temperature drops.
When the oxidative performances of seed and epicotyl mitochondria were monitored throughout a wide range of temperatures, using NADH as a substrate, the seed organelles exhibited an impressive tolerance to extreme conditions, remaining fully active from −3.5°C up to 40°C. In contrast, epicotyl mitochondria had a poor RCR below 0°C and their activities almost collapsed above 30°C. An induction of UCP activity is unlikely to explain the loss of coupling of epicotyl mitochondria at extreme temperatures because mitochondria isolation and assay were performed in the presence of 0.2% bovine serum albumin (BSA). Moreover, even in the presence of a higher concentration of BSA and GTP to ensure full inhibition of UCP activity (Almeida et al., 2002), epicotyl mitochondria still appeared uncoupled at extreme temperatures (data not shown). The overall results highlight the remarkable stress cross-tolerance of seed mitochondria, likely due to specific protection mechanisms built up during seed maturation during the process of desiccation tolerance acquisition. In their seminal work, Lyons and Raison (1970) observed that the occurrence of marked discontinuities (ABTs) in the Arrhenius plot of mitochondrial respiration indicative of Ea changes were a distinctive trait of chilling-sensitive species. Chilling-tolerant species, such as beetroots (Beta vulgaris), potato (Solanum tuberosum) tubers, or cauliflower (Brassica oleracea) buds, exhibited constant Ea over the 1.5°C to 25°C temperature range. In contrast with these observations, we found clear ABTs for both seed and epicotyl mitochondria from pea, which is a chilling-tolerant species. Such a discrepancy might be attributed to the wider temperature range used in our study, which allowed a more accurate determination of ABTs. Although seed and epicotyl mitochondria have contrasting temperature tolerances, their respective ABTs were almost identical, with a change in activation energy occurring at 7°C. According to the homeoviscous adaptation theory, similar biophysical properties might be expected for both types of organelles, which prompted us to examine their lipid and FA compositions. Surprisingly, in spite of the fact that plants were grown at the same optimal temperature, mitochondria from seeds and epicotyls differed in their membrane phospholipid and FA molar compositions. Thus, in addition to the known effect of temperature acclimation on mitochondrial membrane lipid composition reported in plants (De Santis et al., 1999; Davy de Virville et al., 2002) and other species (for review, see Hazel, 1995; Portner, 2002), our results show that development and tissue specificity influence the lipid composition of plant mitochondria. This finding extends an earlier report describing differential lipid composition in spinach (Spinacia oleracea) leaf and petiole mitochondria (Edman and Ericson, 1987). The lower PE/PC molar of seed mitochondria could explain a better performance at low temperatures because it would favor higher fluidity (Hazel, 1995). However, in oat (Avena sativa) and rye (Secale cereale) leaf plasma membrane (Uemura and Steponkus, 1994), as well as in goldfish mitochondria (Van den Thillart and de Bruin, 1981), cold acclimation was associated with a higher PE-to-PC ratio, suggesting that membrane fluidity was not the critical factor determined by the PE-to-PC ratio (Hazel, 1995). PE is often quoted as a nonbilayer lipid because of its propensity to destabilize bilayer lipids and provoke the formation of deleterious inverted hexagonal structures (van den Brink-van der Laan et al., 2004). Since membrane phase transitions are a crucial factor in freezing injury and desiccation tolerance mechanisms (Gordon-Kamm and Steponkus, 1984; Hoekstra et al., 2001), a decreased amount of the destabilizing PE in mitochondrial membranes would be clearly advantageous in the case of seeds. It would prevent the formation of inverted hexagonal bilayer structures in mitochondrial membranes at reduced hydration levels or in the dry state, thus contributing not only to desiccation, but also to freezing tolerance, of the organelle. At the FA level, the significantly lower unsaturation in seed mitochondria should provide the membranes with better heat stability, as has been shown for pea thylakoid membranes (Vigh et al., 1989). In many organisms, the unsaturation level of FAs is well correlated with temperature tolerance (Hazel, 1995), and the link could be neatly demonstrated using genetic and biochemical approaches on cyanobacteria (Vigh et al., 1998). However, lipid and FA composition cannot provide protection at both ends of the temperature scale, and the lower level of unsaturated FAs in seed mitochondria, calling for lower membrane fluidity, is not compatible with their remarkable freezing tolerance. Experimental determination of membrane fluidity will be a prerequisite for exploring the complex link between membrane composition, biophysics, and function of seed mitochondria. The theory of a simple relationship between Arrhenius discontinuities (ABTs) and membrane FA composition has already been challenged by Bligny et al. (1985), who failed to find a correlation between unsaturation level and respiratory properties of sycamore cells. As pointed out, the ABTs could reflect either configurational changes in the enzyme proteins induced by a phase transition in mitochondrial membranes or intrinsic thermotropic changes in protein arrangement independent of lipid fluidity (Bligny et al., 1985).
With respect to abiotic stress, the priority of seeds at the cellular level is to achieve desiccation tolerance, and the low unsaturation level of mitochondrial FAs could have a role in preventing oxidative damage. The management of oxidative stress is indeed intimately linked to seed physiology and deterioration (Bailly, 2004), and PUFAs are especially prone to oxidative attack (Halliwell and Gutteridge, 1999). Mitochondria are indeed especially liable to oxidative stress, as was shown by the modifications of mitochondria biogenesis and function in Arabidopsis (Arabidopsis thaliana) cells subjected to oxidative conditions (Sweetlove et al., 2002). Lipid peroxidation was also shown to interfere with substrate oxidation and target protein modification in pea leaf mitochondria (Taylor et al., 2002). The comparison of isolated mitochondria with respect to lipid peroxidation revealed that seed mitochondria, which have a lower unsaturation level of FAs, were less susceptible than epicotyl mitochondria to lipid peroxidation. A lower unsaturation level in seed mitochondria would thus limit the risk of deleterious lipid peroxidation, which could compromise proper mitochondrial function and hence seed survival. Interestingly, a low degree of FA unsaturation in mitochondrial phospholipids has been correlated with longevity in mammal species (Pamplona et al., 1998). It is therefore tempting to link the lower unsaturation degree in seed mitochondria with the long-term storage ability of seeds. In pea, almost a century of storage is needed to decrease by one-half the initial percentage of germination of accessions stored in seed-storage banks (Walters et al., 2005).
At the protein level, the organelles clearly differ by the strong accumulation of HSP22 and PsLEAm in seed mitochondria, both proteins being likely candidates for stress protection. A general role for small HSPs in desiccation tolerance is suggested by the accumulation of several cytosolic small HSPs during late seed maturation (Wehmeyer and Vierling, 2000). Although the function of HSP22 has not been clearly established, a potential role in the protection of complex I during heat or osmotic stress has been suggested by in vitro experiments (Downs and Heckathorn, 1998; Hamilton and Heckathorn, 2001). The protective role of the protein is illustrated by its ability to increase the thermotolerance of transgenic tobacco (Nicotiana tabacum; Sanmiya et al., 2004) and in Drosophila, where HSP22 was recently discovered, to alleviate oxidative stress and extend life span (Morrow et al., 2004). The HSP17 from Synechocystis, whose expression is closely linked to the physical order of the membrane bilayer, has been shown to associate with the thylakoid membranes (Horvath et al., 1998). Taking into account all these observations, the function of HSP22 in seed mitochondria is likely to be related to the stabilization of macromolecules and possibly membranes, and might contribute, together with lipid composition, to the heat tolerance of pea seed mitochondria. Since LEA proteins frequently figure in the inventory of cold-responsive genes (Thomashow, 1999), the accumulation of PsLEAm within seed mitochondria could be beneficial at low temperatures and/or freezing, thus providing a compensation for the lower unsaturation level in phospholipid FAs. Interestingly, COR15a, a small Arabidopsis cold-responsive protein expected to prevent the transition to the inverse hexagonal phase in thylakoid during freezing, shares with PsLEAm a high hydrophilicity and a predicted amphiphilic helix structure (Steponkus et al., 1998). Further experiments with mitochondria from organisms expressing stress proteins ectopically will be required to ascertain the relative contributions of HSP22 and PsLEAm with respect to heat, cold, and freezing tolerance.
Seeds are the most stress-tolerant form of higher plants, and, accordingly, they were found to harbor mitochondria that appeared extremely resistant to temperature extremes. Such a remarkable stress cross-tolerance is probably driven by the concerted accumulation of stress proteins, such as HSP22 and PsLEAm, and adjustments of biophysical properties of membranes resulting from phospholipids and FA modifications to overcome developmental desiccation. Because respiration is the driving force that powers germination, a high level of mitochondrial stress tolerance certainly contributes to the vigor of germinating seeds in the environment.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Pea (Pisum sativum L. cv Baccara) seeds were grown locally by the agronomical research institute Fédération Nationale des Agriculteurs Multiplicateurs de Semences (FNAMS) and stored in sealed plastic bags at 5°C (70% relative humidity). The seeds used for mitochondrial isolation were imbibed on pleated filter paper in the dark at 20°C for 22 h as described by Benamar et al. (2003). Etiolated seedlings were grown on Perlite for 7 d at 20°C in total darkness. The plant material used for mitochondrial isolation was either the desiccation-tolerant imbibed seeds or the desiccation-sensitive etiolated epicotyls.
Mitochondrial Isolation and Oxygraphic Measurements
Seed mitochondria were isolated from 22-h imbibed seeds and purified using a combination of step and self-generated gradients of Percoll (Amersham Biosciences) described by Benamar et al. (2003). Epicotyl mitochondria were isolated from 7-d-old etiolated seedlings using the same method, except that the isolation buffers contained 0.3 m mannitol (instead of 0.6 m for seed mitochondria) and the step gradient was omitted.
Oxygen consumption of mitochondria was monitored with oxygen electrode systems (Oxytherm and Oxygraph; Hansatech). The solid-state temperature-controlled Oxytherm system was used for assays in the 5°C to 40°C temperature range. The Oxygraph system was connected to a Huber MOD96 cryostat (Peter Huber Kältemaschinenbau GMBH) for temperature control using a 20% (v/v) ethylene glycol fluid circulation. Calibration of the oxygen electrode was performed at each temperature, except for 0°C and −3.5°C, for which the 5°C calibration was used. The electrode medium contained either 0.6 m (for seed) or 0.3 m (for epicotyl) mannitol, 20 mm MOPS (pH 7.5), 10 mm KH2PO4, 10 mm KCl, 5 mm MgCl2, and 0.1% (w/v) BSA. When epicotyl mitochondria were assayed at negative temperatures, the 0.6 m mannitol electrode buffer was used to prevent freezing. Substrates were added at the following final concentrations: succinate (5 mm), malate-Glu (7.5 mm each), and NADH (1.5 mm). Additional cofactors or metabolites required for substrate oxidation were added as required or indicated in the figure legends: 3 mm ATP (for succinate oxidation), 1 mm NAD, 0.3 mm thiamine pyrophosphate, 50 μm CoA, 1 mm pyruvate, and 5 mm dithiothreitol for malate-Glu oxidation. Cyanide (0.2 mm) and 0.2 mm propylgallate were used as inhibitors of electron transfer, and 2 μm carbonyl cyanide FCCP as uncoupler, when required. Outer membrane integrity was measured with cytochrome c as described (Benamar et al., 2003). Protein concentration was determined by a modified Lowry assay (RC DC protein assay; Bio-Rad) using BSA as a standard.
Immunodetection of HSP22 and PsLEAm
Mitochondrial proteins were separated by SDS-PAGE in a discontinuous system (MiniProtean II apparatus; Bio-Rad), using 13.5% (w/v) acrylamide separating gel. Following electrophoresis, the gels were electrophoretically transferred onto nitrocellulose (0.2 μm) membranes (Schleicher and Schuell) for 1 h at 100 V in 25 mm Tris, 192 mm Gly, and 20% (v/v) methanol at pH 8.3 using a mini-transblot system (Bio-Rad). After electroblotting, the membrane was blocked with Tris-buffered saline (TBS) containing 1.5% (v/v) Tween 20 (TBST) for 20 min and rinsed several times with TBS, 0.05% (v/v) Tween 20. The membrane was incubated overnight at 4°C with either a 1/20,000 dilution in TBST of a polyclonal antibody against PsLEAm (accession no. AJ628940; Grelet et al., 2005) or with a 1/1,000 dilution in TBST of a polyclonal antibody raised against an antigenic peptide (MVDLLTDNPVLSAAS) of pea HSP22 (accession no. P46254) produced by Neosystem. The secondary anti-rabbit IgG antibodies (Sigma) conjugated to alkaline phosphatase were used at 1:20,000 dilution. Immunodetection was performed by adding BCIP/NBT-Blue liquid substrate system for membranes (Sigma) to the blot.
Lipid Analysis and Peroxidation Assay
Total lipids were extracted according to Bligh and Dyer (1959) from two pooled aliquots of mitochondrial preparation (totaling 4.5 mg protein). The lipid composition was determined using thin-layer chromatography to separate polar lipids and gas chromatography to analyze their FAs (Dorne et al., 1985). Peroxidation of PUFAs was assayed by measuring MDA and HAE simultaneously using the Bioxytech LPO-586 assay (Oxis International). To examine the effect of oxidative stress, mitochondria suspended in washing medium (0.3 m mannitol, 20 mm KH2PO4, pH 7.2, 0.5 mm EDTA) were supplemented with 500 μm FeSO4 and incubated for 1 h at 25°C prior to the lipid peroxidation assay (Liu et al., 1997).
Acknowledgments
We are especially grateful for the many stimulating discussions with experts in the field who attended the 2005 International Congress on Plant Mitochondrial Biology held in Obernai, France.
This work was supported by a postdoctoral fellowship from the Région Pays-de-la-Loire (to I.S.); by the Contrat de Plan Etat-Région Pays-de-la-Loire, program “Semences”; and by the Russian Fund of Basic Researches (project N 05–04–48966–a).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David Macherel (david.macherel@univ-angers.fr).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.073015.
References
- Almeida AM, Navet R, Jarmuszkiewicz W, Vercesi AE, Sluse-Goffart CM, Sluse FE (2002) The energy-conserving and energy-dissipating processes in mitochondria isolated from wild type and nonripening tomato fruits during development on the plant. J Bioenerg Biomembr 34: 487–498 [DOI] [PubMed] [Google Scholar]
- Atkin OK, Edwards EJ, Loveys BR (2000) Response of root respiration to changes in temperature and its relevance to global warming. New Phytol 147: 141–154 [Google Scholar]
- Atkin OK, Tjoelker MG (2003) Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci 8: 343–351 [DOI] [PubMed] [Google Scholar]
- Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14: 93–107 [Google Scholar]
- Bardel J, Louwagie M, Jaquinod M, Jourdain A, Luche S, Rabilloud T, Macherel D, Garin J, Bourguignon J (2002) A survey of plant mitochondria proteome in relation with development. Proteomics 2: 880–898 [DOI] [PubMed] [Google Scholar]
- Benamar A, Tallon C, Macherel D (2003) Membrane integrity and oxidative properties of mitochondria isolated from imbibing pea seeds after priming or accelerated ageing. Seed Sci Res 13: 35–45 [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bligny R, Rebeillé F, Douce R (1985) O2-triggered changes of membrane fatty acid composition have no effect on Arrhenius discontinuities of respiration in sycamore (Acer pseudoplatanus L.) cells. J Biol Chem 260: 9166–9170 [PubMed] [Google Scholar]
- Borovskii GB, Stupnikova IV, Antipina AI, Downs CA, Voinikov VK (2000) Accumulation of dehydrin-like-proteins in the mitochondria of cold-treated plants. J Plant Physiol 156: 797–800 [Google Scholar]
- Davy de Virville J, Cantrel C, Bousquet A-L, Hoffelt M, Tenreiro A-M, Vaz Pinto V, Arrabaça JD, Caiveau O, Moreau F, Zachowski A (2002) Homeoviscous and functional adaptations of mitochondrial membranes to growth temperature in soybean seedlings. Plant Cell Environ 25: 1289–1297 [Google Scholar]
- De Santis A, Landi P, Genchi G (1999) Changes of mitochondrial properties in maize seedlings associated with selection for germination at low temperature. Fatty acid composition, cytochrome c oxidase, and adenine nucleotide translocase activities. Plant Physiol 119: 743–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorne AJ, Joyard J, Block MA, Douce R (1985) Localization of phosphatidylcholine in outer envelope membrane of spinach chloroplasts. J Cell Biol 100: 1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R, Neuburger M (1989) The uniqueness of plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol 40: 371–414 [Google Scholar]
- Downs CA, Heckathorn SA (1998) The mitochondrial small heat-shock protein protects NADH:ubiquinone oxidoreductase of the electron transport chain during heat stress in plants. FEBS Lett 430: 246–250 [DOI] [PubMed] [Google Scholar]
- Edman K, Ericson I (1987) Phospholipid and fatty acid composition in mitochondria from spinach (Spinacia oleracea) leaves and petioles. Biochem J 243: 575–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Kamm WJ, Steponkus PL (1984) Lamellar-to-hexagonal II phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc Natl Acad Sci USA 81: 6373–6377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelet J, Benamar A, Teyssier E, Avelange-Macherel M-H, Grunwald D, Macherel D (2005) Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. Plant Physiol 137: 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC (1999) Free Radicals in Biology and Medicine, Ed 3. Oxford University Press, New York
- Hamilton EW, Heckathorn SA (2001) Mitochondrial adaptations to NaCl. Complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. Plant Physiol 126: 1266–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazel JR (1995) Thermal adaptation in biological membranes: Is homeoviscous adaptation the explanation? Annu Rev Physiol 57: 19–42 [DOI] [PubMed] [Google Scholar]
- Heazlewood JL, Tonti-Filippini JS, Gout AM, Day DA, Whelan J, Millar AH (2004) Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plant-specific mitochondrial proteins. Plant Cell 16: 241–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra FA, Golovina EA, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6: 431–438 [DOI] [PubMed] [Google Scholar]
- Horvath I, Glatz A, Varvasovszki V, Torok Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, et al (1998) Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci USA 95: 3513–3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnichenko AV, Zykova VV, Grabelnych OI, Sumina ON, Pobezhimova TP, Voinikov VK (2000) Screening of mitochondrial proteins in winter rye, winter wheat, elymus and maize with an immunochemical affinity to the stress protein 310 kD and their intramitochondrial localization in winter wheat. J Therm Biol 25: 245–249 [Google Scholar]
- Lenne C, Block MA, Garin J, Douce R (1995) Sequence and expression of the mRNA encoding HSP22, the mitochondrial small heat-shock protein in pea leaves. Biochem J 311: 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne C, Douce R (1994) A low molecular mass heat-shock protein is localized to higher plant mitochondria. Plant Physiol 105: 1255–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yeo HC, Doniger SJ, Ames BN (1997) Assay of aldehydes from lipid peroxidation: gas chromatography-mass spectrometry compared to thiobarbituric acid. Anal Biochem 245: 161–166 [DOI] [PubMed] [Google Scholar]
- Logan DC, Millar AH, Sweetlove LJ, Hill SA, Leaver CJ (2001) Mitochondrial biogenesis during germination in maize embryos. Plant Physiol 125: 662–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM, Raison JK (1970) Oxidative activity of mitochondria isolated from plant tissues sensitive and resistant to chilling injury. Plant Physiol 45: 386–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar AH, Heazlewood JL, Kristensen BK, Braun HP, Moller IM (2005) The plant mitochondrial proteome. Trends Plant Sci 10: 36–43 [DOI] [PubMed] [Google Scholar]
- Morrow G, Samson M, Michaud S, Tanguay RM (2004) Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J 18: 598–599 [DOI] [PubMed] [Google Scholar]
- Pamplona R, Portero-Otin M, Riba D, Ruiz C, Prat J, Bellmunt MJ, Barja G (1998) Mitochondrial membrane peroxidizability index is inversely related to maximum life span in mammals. J Lipid Res 39: 1989–1994 [PubMed] [Google Scholar]
- Popov VN, Markova OV, Mokhova EN, Skulachev VP (2002) Effects of cold exposure in vivo and uncouplers and recouplers in vitro on potato tuber mitochondria. Biochim Biophys Acta 1553: 232–237 [DOI] [PubMed] [Google Scholar]
- Portner HO (2002) Physiological basis of temperature-dependent biogeography: trade-offs in muscle design and performance in polar ectotherms. J Exp Biol 205: 2217–2230 [DOI] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Stewart CR (1994) Acclimation, hydrogen peroxide, and abscisic acid protect mitochondria against irreversible chilling injury in maize seedlings. Plant Physiol 105: 619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Anderson MD, Stewart CR (1995) Localization and characterization of peroxidases in the mitochondria of chilling-acclimated maize seedlings. Plant Physiol 108: 1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison JK, Lyons JM, Mehlhorn RJ, Keith AD (1971) Temperature-induced phase changes in mitochondrial membranes detected by spin labeling. J Biol Chem 246: 4036–4040 [PubMed] [Google Scholar]
- Rasmusson AG, Soole KL, Elthon TE (2004) Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu Rev Plant Biol 55: 23–39 [DOI] [PubMed] [Google Scholar]
- Sanmiya K, Suzuki K, Egawa Y, Shono M (2004) Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. FEBS Lett 557: 265–268 [DOI] [PubMed] [Google Scholar]
- Sinensky M (1974) Homeoviscous adaptation. A homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci USA 71: 522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M, Bowles DJ (2002) Plants in a cold climate. Philos Trans R Soc Lond B Biol Sci 357: 831–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steponkus PL, Uemura M, Joseph RA, Gilmour SJ, Thomashow MF (1998) Mode of action of the COR15a gene on the freezing tolerance of Arabidopsis thaliana. Proc Natl Acad Sci USA 95: 14570–14575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DY, Kaplan F, Lee KJ, Guy CL (2003) Acquired tolerance to temperature extremes. Trends Plant Sci 8: 179–187 [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Heazelwood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH (2002) The impact of oxidative stress on Arabidopsis mitochondria. Plant J 32: 891–904 [DOI] [PubMed] [Google Scholar]
- Taylor NL, Day DA, Millar AH (2002) Environmental stress causes oxidative damage to plant mitochondria leading to inhibition of glycine decarboxylase. J Biol Chem 277: 42663–42668 [DOI] [PubMed] [Google Scholar]
- Taylor NL, Heazlewood JL, Day DA, Millar AH (2005) Differential impact of environmental stresses on the pea mitochondrial proteome. Mol Cell Proteomics 4: 1122–1133 [DOI] [PubMed] [Google Scholar]
- Taylor NL, Rudhe C, Hulett JM, Lithgow T, Glaser E, Day DA, Millar AH, Whelan J (2003) Environmental stresses inhibit and stimulate different protein import pathways in plant mitochondria. FEBS Lett 547: 125–130 [DOI] [PubMed] [Google Scholar]
- Thomashow MF (1999) Plant cold acclimation, freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50: 571–599 [DOI] [PubMed] [Google Scholar]
- Uemura M, Steponkus PL (1994) A contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiol 104: 479–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brink-van der Laan E, Killian JA, de Kruijff B (2004) Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta 1666: 275–288 [DOI] [PubMed] [Google Scholar]
- Van den Thillart G, de Bruin G (1981) Influence of environmental temperature on mitochondrial membranes. Biochim Biophys Acta 640: 439–447 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L (1997) Alternative oxidase, from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48: 703–734 [DOI] [PubMed] [Google Scholar]
- Vigh L, Gombos Z, Horvath I, Joo F (1989) Saturation of membrane lipids by hydrogenation induces thermal stability in chloroplast inhibiting the heat-dependent stimulation of photosystem I-mediated electron transport. Biochim Biophys Acta 979: 361–364 [Google Scholar]
- Vigh L, Maresca B, Harwood JL (1998) Does the membrane's physical state control the expression of heat shock and other genes? Trends Biochem Sci 23: 369–374 [DOI] [PubMed] [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM (2005) Longevity of seeds stored in a genebank: species characteristics. Seed Sci Res 15: 1–20 [Google Scholar]
- Wehmeyer N, Vierling E (2000) The expression of small heat shock proteins in seeds responds to discrete developmental signals and suggests a general protective role in desiccation tolerance. Plant Physiol 122: 1099–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]