Abstract
The reaction of hypochlorous acid (HOCl) with hydrogen peroxide is known to generate stoichiometric amounts of singlet molecular oxygen [O2 (1Δg)]. This study shows that HOCl can also react with linoleic acid hydroperoxide (LAOOH), generating O2 (1Δg) with a yield of 13 ± 2% at physiological pH. Characteristic light emission at 1,270 nm, corresponding to O2 (1Δg) monomolecular decay, was observed when HOCl was reacted with LAOOH or with liposomes containing phosphatidylcholine hydroperoxides, but not with cumene hydroperoxide or tert-butyl hydroperoxide. The generation of O2 (1Δg) was confirmed by the acquisition of the spectrum of the light emitted in the near-infrared region showing a band with maximum intensity at 1,270 nm and by the observation of the enhancing effect of deuterium oxide and the quenching effect of sodium azide. Mechanistic studies using 18O-labeled linoleic acid hydroperoxide (LA18O18OH) showed that its reaction with HOCl yields 18O-labeled O2 (1Δg) [18O2 (1Δg)], demonstrating that the oxygen atoms in O2 (1Δg) are derived from the hydroperoxide group. Direct analysis of radical intermediates in the reaction of LAOOH with HOCl by continuous-flow electron paramagnetic resonance spectroscopy showed a doublet signal with a g-value of 2.014 and a hyperfine coupling constant from the α-hydrogen of aH = 4.3 G, indicating the formation of peroxyl radicals. Taken together, our results clearly demonstrate that HOCl reacts with biologically relevant lipid hydroperoxides, generating O2 (1Δg). In addition, the detection of 18O2 (1Δg) and peroxyl radicals strongly supports the involvement of a Russell mechanism in the generation of O2 (1Δg).
Keywords: lipid hydroperoxides, mass spectrometry, myeloperoxidase, near-infrared emission, 18O-labeled oxygen
Hypochlorous acid (HOCl) is a potent oxidant generated in neutrophils by the reaction of chloride ion with hydrogen peroxide (Eq. 1) catalyzed by myeloperoxidase (MPO) (1-4). This heme enzyme is stored at high concentrations in the granules of phagocytic cells (neutrophils and monocytes), and, upon stimulation, it is secreted into both the extracellular milieu and the phagocytic vacuole (3). It is believed that the generation of HOCl by this system constitutes an important defense mechanism against microorganisms (3, 4). However, excessive production of HOCl can also lead to host tissue injury (5), contributing to the development of several diseases, such as atherosclerosis (6, 7) and cancer (8).
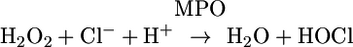 |
[1] |
At physiological pH, HOCl is in equilibrium with its conjugate base, hypochorite (OCl-, pKa 7.4 at 25°C) (9), (Eq. 2), and both forms appear to be responsible for the oxidation and/or halogenation reactions. It has also been demonstrated that, at acidic conditions, HOCl can be in equilibrium with molecular chlorine (Cl2,pKa 3.3) (10) through a reaction that requires Cl- and H+ (Eq. 3) (11). In vitro studies suggest that Cl2 might be the chlorinating agent that mediates the formation of chlorinated products during phagocytosis (11-13).
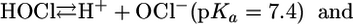 |
[2] |
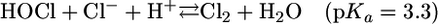 |
[3] |
HOCl is a highly reactive species capable of modifying a variety of biomolecules (5). Free amino and thiol groups of amino acids and peptides constitute important targets for HOCl, yielding unstable chloramines and sulfenyl chloride intermediates, respectively (14-16). Chloramine intermediates are also detected in the reaction of HOCl with exocyclic (RNH2) and heterocyclic (RNHR) amine functions in DNA bases (14, 17). HOCl reacts with aromatic rings, such as in tyrosine, yielding 3-chlorotyrosine and 3,5-dichlorotyrosine (5, 18, 19). These products have been detected in proteins exposed to MPO or stimulated neutrophils (20) and in low-density lipoprotein isolated from atherosclerotic lesions (19).
Another important target for HOCl is lipids. It is known that HOCl adds across the carbon-carbon double bonds in fatty acids and cholesterol, yielding chlorohydrins (6, 21, 22). HOCl has been shown to induce lipid peroxidation (23). Several groups have detected accumulation of lipid-peroxidation products in liposomes and lipoproteins after incubation with HOCl or MPO-H2O2-Cl- system (24-26). However, the mechanism by which HOCl induces lipid peroxidation remains unclear. The Panasenko group (23, 26, 27) postulated that the presence of lipid hydroperoxides is critical for the initiation of lipid peroxidation in these systems. They have proposed that the reaction of HOCl with lipid hydroperoxides yields free radicals able to cause further oxidation of lipid molecules (27).
It is well known that HOCl reacts with hydrogen peroxide, yielding stoichiometric amounts of singlet molecular oxygen [O2 (1Δg)] (Eq. 4) (28-30). However, little is known about the reaction of HOCl with other biologically relevant hydroperoxides, such as lipid hydroperoxides, which are the primary products of lipid peroxidation and are also generated in lipoxygenase- and ciclooxygenase-catalyzed reactions (31).
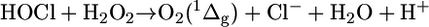 |
[4] |
The aim of this study was to investigate whether O2 (1Δg) can be generated during the reaction of HOCl with lipid hydroperoxides. We have used hydroperoxides derived from linoleic acid (LAOOH), one of the major fatty acids present in biological systems, and liposomes enriched with phosphatidylcholine hydroperoxides (PCOOH). The generation of O2 (1Δg) was studied by direct spectroscopic detection and characterization of O2 (1Δg) monomol emission at the near-infrared region. The reaction mechanism was investigated by measuring the formation of 18O-labeled O2 (1Δg) [18O2 (1Δg)] in the reaction of 18O-labeled linoleic acid hydroperoxide (LA18O18OH) with HOCl by chemical trapping and mass-spectrometry analysis and of radical intermediates by rapid-mixing continuous-flow electron paramagnetic resonance spectroscopy (EPR).
Results
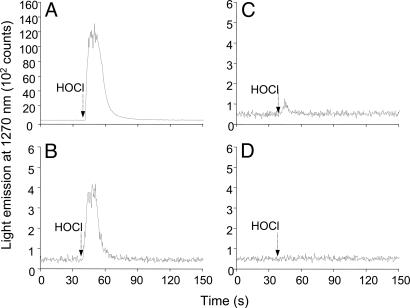
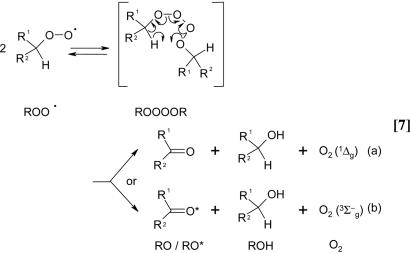
Singlet Oxygen Generation in the Reaction of HOCl with LAOOH. The generation of O2 (1Δg) in the reaction of HOCl with LAOOH was investigated by monitoring the light emission at 1,270 nm corresponding to O2 (1Δg) monomolecular decay (1Δg → 3Σg-) (Eq. 5). For comparison, the light emission for the reaction of HOCl with other hydroperoxides, such as H2O2, cumene hydroperoxide (CuOOH), and tert-butylhydroperoxide (t-BuOOH) were also studied (Fig. 1). As expected, an intense light emission was observed upon injection of HOCl (final concentration, 1 mM) into a solution of H2O2 (1 mM prepared in D2O), consistent with the stoichiometric generation of O2 (1Δg) in this reaction (Fig. 1A) (28-30). Similarly, the injection of HOCl (final concentration, 1 mM) into a solution of LAOOH (1 mM solubilized in 0.1 M phosphate buffer, pH 7.4, in D2O) also produced a rapid increase in light emission whose intensity was ≈30 times lower compared with the reaction with H2O2 (Fig. 1B). In contrast, the injection of HOCl into a solution containing t-BuOOH showed a very small light emission (Fig. 1C), and the injection of HOCl into a solution of CuOOH did not yield any light emission (Fig. 1D). These results indicate that the reaction of HOCl with secondary hydroperoxides, such as LAOOH, generates O2 (1Δg), whereas the reaction with tertiary hydroperoxides does not yield O2 (1Δg). The small emission signal observed upon reaction of HOCl with t-BuOOH is probably due to contaminant H2O2 normally present in commercial t-BuOOH.
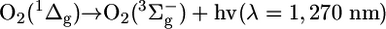 |
[5] |
Fig. 1.
Monomol light emission of O2 (1Δg) generated in the reaction of HOCl with different hydroperoxides. Light emission observed upon injection of 0.17 ml of 10 mM HOCl (final concentrationm, 1 mM) into 1.5 ml of 1 mM H2O2 (A), 1 mM LAOOH (B), 1 mM t-BuOOH (C), and 1 mM CuOOH (D).
The amount of O2 (1Δg) generated in the reaction of HOCl with LAOOH was estimated by the integration of the light-emission signal at 1,270 nm by using DHPNO2, a water-soluble O2 (1Δg) generator, as a reference (see Fig. 8, which is published as supporting information on the PNAS web site). When 1 mM of LAOOH was reacted with various concentrations of HOCl, a concentration-dependent increase in the amount of O2 (1Δg) was observed from 0.2 to 2.0 mM HOCl, reaching a plateau >2.0 mM (see Fig. 9, which is published as supporting information on the PNAS web site). The maximum concentration of O2 (1Δg) at the plateau was 133 ± 16 μM, which corresponds to a yield of 13 ± 2% of O2 (1Δg).
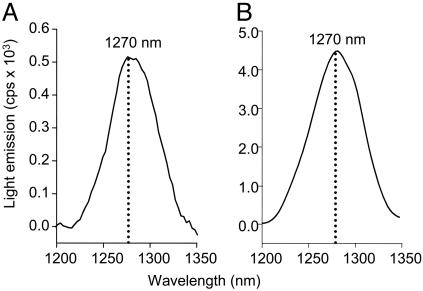
Singlet-Oxygen Spectrum in the Near-Infrared Region. The generation of O2 (1Δg) by the reaction of HOCl with LAOOH was also confirmed by recording the spectrum of the light emitted in the near-infrared region (Fig. 2A). For comparative purposes, the spectrum of the O2 (1Δg) generated by H2O2 with HOCl was also acquired (Fig. 2B). Both spectra showed an emission band with maximum intensity at 1,270 nm, characteristic of O2 (1Δg) monomolecular decay. Further evidences that the light emitted in the reaction corresponds to O2 (1Δg) were obtained by testing the effect of nondeuterated vs. deuterated solvent and the effect of NaN3, a known O2 (1Δg) quencher (32) (see Fig. 10, which is published as supporting information on the PNAS web site). The intensity of the light emitted in the reaction in 100% D2O was about eight times higher than in the reaction in 12% D2O, consistent with the longer lifetime of O2 (1Δg) in D2O than in H2O (33, 34) (Fig. 10A). The quenching effect of 1 mM of NaN3 (75% inhibition) on the chemiluminescent reaction of HOCl with LAOOH was also observed (Fig. 10B).
Fig. 2.
Monomol light-emission spectrum of O2 (1Δg) generated in the reaction of HOCl with LAOOH (A) and H2O2 (B).
Light-Emission Measurements in the Reaction of HOCl with Linoleic Acid (LA) or Hydroxy Linoleate (LAOH). To assess whether the hydroperoxyl group of LA is essential for the generation of O2 (1Δg), we have performed experiments with LA and LAOH (see Fig. 11, which is published as supporting information on the PNAS web site). No emission was observed when HOCl was injected into a solution containing LA, whereas a small emission was observed in the reaction of HOCl with LAOH. Analysis by mass spectrometry showed that this emission is due to the presence of 10% residual LAOOH in the solution of LAOH (see Fig. 12, which is published as supporting information on the PNAS web site).
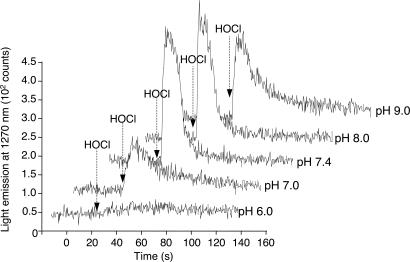
Effect of pH on Singlet-Oxygen Generation in the Reaction of HOCl with LAOOH. The generation of O2 (1Δg) in the reaction of H2O2 with HOCl is known to vary with pH, showing higher yields at alkaline pH (29). To check whether a similar pH profile is also observed in the reaction of LAOOH with HOCl, we have measured the light emission during the injection of HOCl into a solution containing LAOOH dispersed in phosphate buffer at various pHs (Fig. 3). The light emission was very intense at pH 7.4 and pH 8.0, decreasing at pH 7.0 and pH 9.0. Considering that the pKa of HOCl is 7.4, these results suggest that the generation of O2 (1Δg) is favored by the presence of the anionic form of HOCl.
Fig. 3.
Effect of pH on O2 (1Δg) generation in the reaction of LAOOH with HOCl. Reaction was initiated by injection of 0.17 ml of 10 mM HOCl (final concentration, 1 mM) into 1.5 ml of 1 mM LAOOH solution in 0.1 M phosphate buffer prepared in D2O at the following pHs 6.0, 7.0, 7.4, 8.0, and 9.0.
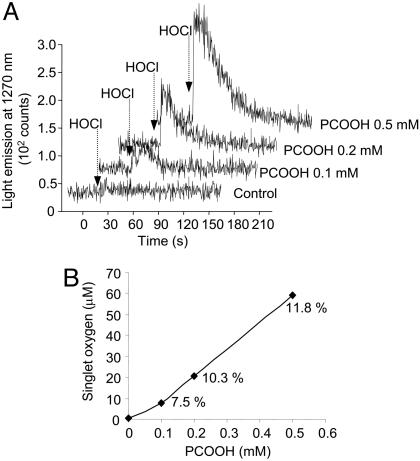
Singlet-Oxygen Generation upon Reaction of HOCl with Phospholipid Hydroperoxides in Liposomes. To investigate whether HOCl can also interact with lipid hydroperoxides present in membranes, we have done experiments with unilamellar liposomes containing different concentrations of PCOOH. The presence of increasing amounts of PCOOH in the membrane led to an almost linear increase in the intensity of the light emitted upon injection of HOCl (Fig. 4). Interestingly, the estimated yield of O2 (1Δg) was augmented at higher concentrations of PCOOH in the membrane. This result shows that the HOCl can also react with phospholipid hydroperoxides present in membranes to generate O2 (1Δg).
Fig. 4.
Monomol light emission of O2 (1Δg) observed during injection of HOCl into PC liposomes containing different concentrations of PCOOH. (A) Light emission monitored during injection of 0.17 ml of 100 mM HOCl (final concentration 10 mM) into 1.5 ml of 1 mM PC vesicles containing 0, 10, 20, or 50% PCOOH. (B) The amount of O2 (1Δg) calculated by integration of the area under emission signal. The percentages indicate the yield of O2 (1Δg).
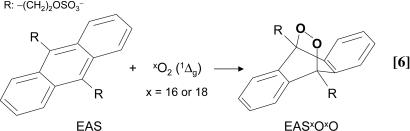
Detection of 18O-Labeled Singlet Oxygen in the Reaction of LA18O18OH with HOCl. To characterize the mechanism involved in the generation of O2 (1Δg) by the reaction of HOCl with LAOOH, we used LA18O18OH as a mechanistic tool. Singlet oxygen generated by the reaction of HOCl with LAOOH or LA18O18OH was chemically trapped with the anthracene derivative, anthracene-9,10-diyldiethyl sulfate (EAS) (Eq. 6) and the corresponding endoperoxides (EASxOxO, x = 16 or 18), were detected by HPLC coupled to tandem MS (HPLC-MS/MS), as reported in ref. 35.
Figure 6.
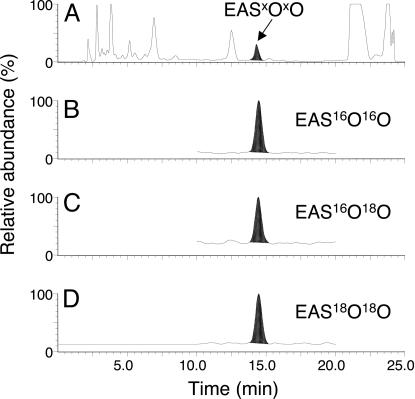
The reaction of HOCl with LA18O18OH showed the generation of a mixture of three endoperoxides, namely, the completely labeled endoperoxide (EAS18O18O), an endoperoxide containing 18O and 16O atoms (EAS16O18O), and an unlabeled endoperoxide (EAS16O16O). Fig. 5 shows the typical chromatograms for EASxOxO analysis by UV and mass detection. Analysis of the products by UV at 215 nm showed a peak corresponding to the endoperoxide at 14.5 min and a peak corresponding to EAS at 19 min. The mass chromatograms obtained by selecting ions with m/z 228, 229, and 230 shows the presence of EAS16O16O, EAS16O18O, and EAS18O18O, respectively. The identity of the ions was confirmed by analyzing the mass spectra of the daughter ions derived from each endoperoxide (see Fig. 13, which is published as supporting information on the PNAS web site).
Fig. 5.
Analysis of EAS endoperoxides formed in the reaction of LA18O18OH (5 mM) with HOCl (10 mM) in the presence of EAS (8 mM) by HPLC-MS/MS. (A) HPLC chromatogram monitored at UV 215 nm. Mass chromatograms obtained by selecting the ions at m/z 228 (B), m/z 229 (C), and 230 (D).
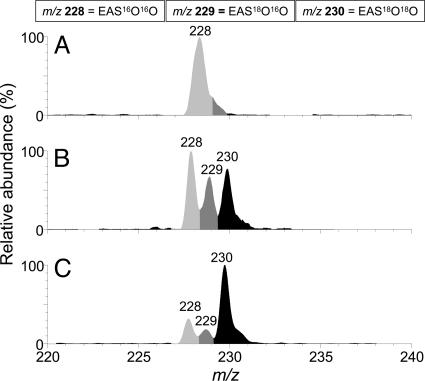
In other studies, we have observed that oxygen affects the detection of 18O2 (1Δg) generated in the reaction of LA18O18OH with metal ions (36) or during thermolysis of an 18O-labeled naphthalene endoperoxide (35). To determine whether oxygen interferes in the detection of 18O2 (1Δg) in this reaction, we have conducted experiments in the presence and absence of oxygen. Fig. 6 shows the relative intensities of the endoperoxides formed in the reaction of HOCl with unlabeled hydroperoxide (LA16O16OH) or with LA18O18OH. As expected, the reaction of HOCl with LA16O16OH yielded a prominent ion at m/z 228, corresponding to EAS16O16O (Fig. 6A). In contrast, the reaction of HOCl with LA18O18OH conducted in the presence of oxygen showed the formation all three endoperoxides, as observed by the presence of ions at m/z 228, 229, and 230 in the proportion of 40:28:32 (Fig. 6B). Removal of the oxygen from the reaction of HOCl with LA18O18OH by a repetitive procedure of freezing and thawing under vacuum led to an expressive increase in the amount of EAS18O18O and decrease in the amount of EAS16O16O and EAS16O18O (Fig. 6C). The proportion of EAS16O16O, EAS16O18O, and EAS18O18O detected after removal of oxygen was 21:17:62.
Fig. 6.
Electrospray ionization (ESI)-MS spectrum of the EAS endoperoxides formed by the reaction of LAxOxOH (x = 16 or 18) with HOCl. (A) LA16O16OH (5 mM) was reacted with 10 mM HOCl in 0.1 M phosphate buffer, pH 7.4. (B) LA18O18OH (5 mM) was reacted with 10 mM HOCl in 0.1 M phosphate buffer, pH 7.4 in 85% D2O, 10% H2O, and 5% MeOH. (C) Reaction described in B without oxygen.
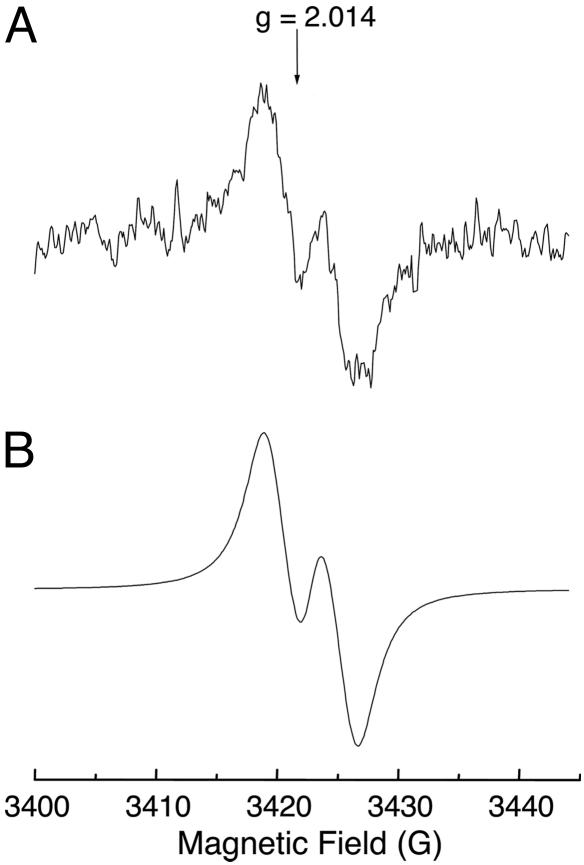
Detection of Peroxyl Radical by Continuous-Flow EPR. As recently confirmed by our group, O2 (1Δg) can be generated by the combination of peroxyl radical, by following the mechanism described by Russell (37). To determine whether a similar type of reaction is involved in the generation of O2 (1Δg) by the reaction of HOCl with LAOOH, we studied whether peroxyl radicals are formed in this reaction. Fig. 7 shows the experimental and simulated EPR spectra acquired for the reaction of HOCl with LAOOH. Continuous infusion of concentrated solutions of LAOOH (final concentration, 14 mM) and HOCl (final concentration, 14 mM) in phosphate buffer, pH 7.4, containing 25% acetonitrile at room temperature produced a broad doublet signal with a distinctive g-value of 2.014 that is characteristic of peroxyl radicals (Fig. 7A) (38-41). The simulated spectrum showed a hyperfine coupling constant of 4.3 G (Fig. 7B), probably due to the interaction of the radical with the allylic hydrogen. A similar coupling constant was observed by Chamulitrat and Mason (41) during the oxidation of arachidonic acid by lipoxygenase. Thus, this result proves that the reaction of HOCl with LAOOH generates peroxyl radicals.
Fig. 7.
EPR spectrum of linoleate peroxyl radicals. (A) Experimental spectrum obtained during the reaction of HOCl with LAOOH. Spectrometer settings were microwave frequency, 9.650 GHz; microwave power, 10 mW; field-modulation frequency, 100 kHz; field-modulation amplitude, 3 G; receiver gain, 1 × 106; time constant, 164 ms; scan rate, 1.2 G s-1; number of scans, 1. (B) Computer simulation of spectrum A using the following values: aH = 4.3 G; line width, 2.8 G; center of the spectrum, 3,423 G.
Discussion
It is well established that HOCl reacts with hydrogen peroxide to yield stoichiometric amounts of O2 (1Δg) (28-30). Our results show that HOCl can also react with lipid hydroperoxides, such as fatty acid hydroperoxides or phosphatidylcholine hydroperoxides contained in liposomes, to yield O2 (1Δg) at physiological pH. The formation of O2 (1Δg) in the reaction of HOCl with LAOOH was clearly demonstrated by the direct detection and characterization of the O2 (1Δg) monomol emission at 1,270 nm.
The generation of O2 (1Δg) was also tested with tertiary hydroperoxides, t-BuOOH or Cu-OOH. The reaction of HOCl with these hydroperoxides did not yield O2 (1Δg), suggesting that the presence of a hydrogen-α at the carbon to which the hydroperoxide is attached is essential for the generation of O2 (1Δg). The presence of hydrogen-α is known to be critical for the generation of O2 (1Δg) by the Russell mechanism (Eq. 7) (37). In this mechanism, primary or secondary peroxyl radicals (ROO•) react by a cyclic mechanism involving a linear tetraoxide intermediate (ROOOOR) that decomposes to generate a ketone (RO), an alcohol (ROH), and oxygen (36, 37, 42). It has been postulated that this reaction may generate either an electronically excited oxygen molecule (Eq. 7a) or an electronically excited ketone (Eq. 7b). Niu and Mendenhall (43) reported that the yields of O2 (1Δg), in the case of simple alkylhydroperoxides, ranged from 3.9% to 14.0%. In contrast, the yields of excited carbonyls were 103 to 104 lower, suggesting that the self-reaction of peroxyl radical generates predominantly O2 (1Δg).
Figure 9.
The mechanism involved in the generation of O2 (1Δg) by the reaction of HOCl with LAOOH was studied by using 18O-labeled hydroperoxide. The reaction of LA18O18OH with HOCl in the presence of EAS yielded a mixture of endoperoxides containing 18O and/or 16O atoms (EAS16O16O, EAS16O18O, and EAS18O18O). Comparison of the relative amounts of EAS16O16O/EAS16O18O/EAS18O18O detected before and after removal of oxygen showed an expressive increase in the amount of EAS18O18O and decrease in the amount of both EAS16O16O and EAS18O16O. These results indicate that oxygen affects the detection of 18O2 (1Δg). It can be also concluded that the reaction of HOCl with LA18O18OH yields mainly 18O2 (1Δg) and that the oxygen atoms in the 18O2 (1Δg) molecule are derived from the hydroperoxide moiety.
The influence of oxygen in the detection of 18O2 (1Δg) may be explained by two mechanisms. One is an energy-transfer mechanism between 18O2 (1Δg) and 16O2 (3Σg-), yielding 16O2 (1Δg) and 18O2 (3Σg-), as recently demonstrated for aqueous systems by Martinez et al. (35). Another mechanism takes into account the generation of 18O-labeled peroxyl radicals (LA18O18O•) in the reaction of HOCl with LA18O18OH as precursors of 18O2 (1Δg). As proposed by Chan (44), the labeled oxygen atoms in LA18O18O• can exchange with the surrounding 16O2, yielding unlabeled peroxyl radicals (LA16O16O•). Accordingly, the formation of LA16O16O• may explain the formation of 16O2 (1Δg) and the formation of O2 (1Δg) containing a mixture of 16O and 18O atoms, in the presence of oxygen.
On the basis of 18O2 (1Δg) detection in the reaction of HOCl with LA18O18OH, we have studied the possible mechanisms involved in its generation. The generation of 18O2 (1Δg) could occur through a mechanism similar to the reaction of HOCl with H2O2. Cahil and Taube (45), using 18O-labeled hydroperoxide (H18O18OH), demonstrated that the oxygen atoms in the oxygen molecule generated by the reaction of HOCl with H2O2 were completely labeled. The mechanism proposed for this reaction involves a nucleophilic attack of HO2- on the chlorine atom of HOCl to form a [HOO-Cl-OH]- intermediate, which then generates O2 (1Δg) by a two-electron transfer (Eq. 8) (29). In analogy, we could suggest the possibility of a nucleophilic attack of LA18O18O- on HOCl to yield a [LA18O18O-Cl-OH] intermediate that decomposes, generating 18O2 (1Δg) (Eq. 9). However this mechanism does not explain the requirement of a hydrogen-α in the hydroperoxide structure for the formation of O2 (1Δg) and the detection of O2 (1Δg), containing a mixture of 16O and 18O atoms.
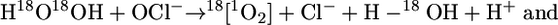 |
[8] |
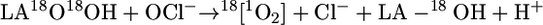 |
[9] |
An alternative mechanism by which the formation of 18O2 (1Δg) in the reaction of HOCl with LA18O18OH could be explained is the Russell mechanism (Eq. 7) (37). This mechanism requires the generation of 18O-labeled LA peroxyl radicals (LA18O18O•). Indeed, peroxyl-radical intermediates were directly detected in the reaction of HOCl with LAOOH by continuous-flow EPR.
The detection of peroxyl radicals, the requirement of a hydrogen-α in the hydroperoxide structure and the O2 (1Δg) yield of 13 ± 2%, which is very close to the estimated yield of a Russell mechanism, strongly points to the involvement of the Russell mechanism in the generation of O2 (1Δg) by the reaction of HOCl with LAOOH. However, the formation of peroxyl radical by direct one-electron oxidation of LAOOH by HOCl is thermodynamically unfavorable. HOCl is a relatively poor one-electron oxidant, having an estimated one-electron reduction potential at pH 7 (E°′) in the range of 0.17-0.25 V (46) and, therefore, not able to promote the one-electron oxidation of LAOOH to LAOO•, which has a reduction potential of 1.0 V (47, 48).
Alternatively, peroxyl radicals could be generated by a mechanism involving chlorine-atom transfer between HOCl and LAOOH. This type of reaction is reported to occur in the reaction of HOCl with NO2-, yielding a NO2Cl intermediate (49) that decomposes, generating Cl• and •NO2 (50). Similar reactions also occur during interaction of HOCl with thiols (RSH) and amines (RNH2), yielding the corresponding chlorinated products RSCl and RNHCl. These intermediates are relatively unstable and are suggested to decompose thermally or in the presence of metal ions to generate the radical intermediates RS• and Cl• (51) or RNH• and Cl• (52, 53).
Analogous to the reactions described above, a chlorine-atom transfer from HOCl to LAOOH could yield unstable chlorinated peroxide intermediates (LAOOCl) (Eq. 10) that may undergo homolytic cleavage to generate LAO• and •OCl (Eq. 11) or LAOO• and Cl• (Eq. 12). The Cl• radical is a strong oxidant (E° Cl•/Cl = 2.2-2.6 V) (54) capable of promoting the direct oxidation of LAOOH to LAOO•. In the same way, the alkoxyl radical LAO•, which has a reduction potential of 1.6 V (47, 48), could also oxidize LAOOH to LAOO•.
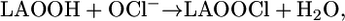 |
[10] |
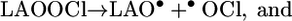 |
[11] |
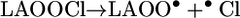 |
[12] |
Another mechanism by which HOCl could promote the formation of peroxyl radicals is the reaction with O2•- or Fe2+, generating •OH (55-57). The hydroxyl radical is considered to be one of the most powerful oxidants, with E°′ •OH, H/H2O = 2.31 V (58) and, therefore, capable of oxidizing LAOOH to LAOO•. However, the involvement of contaminant metal ions or •OH in our system can be discarded, because experiments done with Chelex-treated buffer and manitol (1-10 mM) did not affect the formation of O2 (1Δg)by the reaction of HOCl with LAOOH (data not shown).
Overall, our results clearly demonstrated that HOCl reacts with both fatty acid hydroperoxides and phospholipid hydroperoxides present in membranes to generate O2 (1Δg). Moreover, our study also provided direct evidence for the generation of lipid peroxyl-radical intermediates in the reaction of HOCl with LAOOH. The physiological relevance of these findings remains to be clarified. Nonetheless, the generation of both lipid peroxyl radicals and O2 (1Δg) in this reaction may be an additional important mechanism that contributes to the microbicidal activity of HOCl during phagocytosis as well as for the propagation of lipid peroxidation in pathologic conditions involving inflammatory processes, such as atherosclerosis and cancer. As recently reviewed, O2 (1Δg) is a highly reactive species that can oxidize a variety of biomolecules (59) and can modulate cell-signaling cascades and gene expression (60).
The importance of our study is strengthened by a number of evidences indicating a link between inflammatory disorders to lipid peroxidation and the formation of biologically active oxidized lipids (24, 61, 62). It has been demonstrated that MPO functions as a major catalyst for initiation of lipid peroxidation at sites of inflammation (61, 62). Our findings add a pathway that can contribute to the promotion of lipid peroxidation, providing further insights into the potential involvement of O2 (1Δg) in oxidative reactions mediated by HOCl/lipid hydroperoxides in biological systems.
Conclusions
In summary, the results presented in this article clearly show that the reaction of HOCl with LAOOH or PCOOH generates O2 (1Δg). The requirement of a hydrogen-α for the generation of O2 (1Δg), the formation of 18O2 (1Δg) in the reaction of HOCl with LA18O18OH, and the direct detection of peroxyl radicals by EPR, strongly suggest the involvement of a Russell mechanism. The generation of O2 (1Δg) by the reaction of HOCl with lipid hydroperoxides may be another important reaction that occurs at sites of inflammation.
Materials and Methods
Materials. LA, egg-yolk phosphatidylcholine (PC), and sodium azide were obtained from Sigma. Deuterium oxide (D2O, 99.9%) was from Aldrich (Steinheim, Germany). H2O2 was purchased from Peróxidos do Brasil (Paraná, Brazil). HOCl stock solution (≈0.2-0.4 M) was prepared by vacuum distillation at 40°C of commercial hypochlorite solution acidified to pH 6 with phosphoric acid. The HOCl concentration was determined spectrophotometrically (ε292 = 350 M-1·cm-1 at pH 12) (63). LA18O18OH and PCOOH were synthesized as described in refs. 36 and 64. The disodium salt of anthracene, EAS, and the endoperoxide of N,N′-di(2,3-dihydroxypropyl)-1,4-naphthalenedipropionamide (DHPNO2) were synthesized as described by Di Mascio and Sies (65) and Martinez et al. (66), respectively. All of the other solvents were of HPLC grade and were acquired from Merck.
Singlet-Oxygen Monomol Light-Emission Measurements. Monomolecular photoemission of O2 (1Δg) at 1270 nm was monitored by a photocounting apparatus described in refs. 36 and 67. For details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Singlet-Oxygen Spectrum in the Infrared Region. The spectrum of the light emitted in the near-infrared region was recorded with the photomultiplier described above. For details, see Supporting Materials and Methods.
Calculation of Singlet-Oxygen Yield. The yield of O2 (1Δg) was calculated by using DHPNO2, a water-soluble endoperoxide, as a clean source of O2 (1Δg) (see Supporting Materials and Methods and Fig. 8).
Preparation of Liposomes Containing Phospholipid Hydroperoxides. Liposomes of defined size (100 nm) were prepared by an extrusion technique (64). For details, see Supporting Materials and Methods.
18O-Labeled Singlet-Oxygen Detection by Chemical Trapping. Singlet oxygen generated in the reaction of LA18O18OH with HOCl was chemically trapped with EAS. EASO2 was analyzed by HPLC-MS/MS. Details of the method are described in Supporting Materials and Methods.
epr. EPR spectra of transient species formed at room temperature (25 ± 2°C) were obtained with an EMX spectrometer equipped with a ER 4117 D-MVT dielectric-mixing resonator (Bruker, Billerica, MA) (68). The magnetic field was calibrated with 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) which has a g-value of 2.0056 (69). Computer simulations of spectra were performed by using the program winsim (EPR calculations for MS-Windows NT 95, version 0.96 from Public EPR Software Tools (P.E.S.T.) written by Duling (70). For details, see Supporting Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by the Brazilian research funding institutions Fundação de Amparo `a Pesquisa do Estado de São Paulo, Conselho Nacional de Pesquisa, Instituto do Milênio: Redoxoma, and The John Simon Memorial Guggenheim Foundation (P.D.M., Fellow).
Author contributions: S.M., O.A., M.H.G.M., and P.D.M. designed research; S.M. and G.R.M. performed research; S.M., G.R.M., and D.R. contributed new reagents/analytic tools; S.M., G.R.M., O.A., M.H.G.M., and P.D.M. analyzed data; and S.M., O.A., and P.D.M. wrote the paper.
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EAS, anthracene-9,10-diyldiethyl sulfate; EPR, electron paramagnetic resonance spectroscopy; HOCl, hypochlorous acid; LA, linoleic acid; LAOOH, LA hydroperoxide; O2 (1Δg), singlet molecular oxygen; PCOOH, phosphatidylcholine hydroperoxide; MPO, myeloperoxidase; t-BuOOH, tert-butylhydroperoxide.
References
- 1.Allen, R. C. (1994) Environ. Health Perspect. 102, 201-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison, J., Watson, B. & Schultz, J. (1978) FEBS Lett. 92, 327-332. [DOI] [PubMed] [Google Scholar]
- 3.Klebanoff, S. J. (1999) in Inflammation: Basic Principles and Clinical Correlates, eds. Gallin, J. I. & Snyderman, R. (Lippincott, Philadelphia), pp. 721-768.
- 4.Hampton, M., Kettle, A. & Winterbourn, C. (1998) Blood 92, 3007-3017. [PubMed] [Google Scholar]
- 5.Winterbourn, C. C. & Kettle, A. J. (2000) Free Radical Biol. Med. 29, 403-409. [DOI] [PubMed] [Google Scholar]
- 6.Heinecke, J. W. (1994) Coronary Artery Dis. 5, 205-210. [DOI] [PubMed] [Google Scholar]
- 7.Daugherty, A., Dunn, J. L., Rateri, D. L. & Heinecke, J. W. (1994) J. Clin. Invest. 94, 437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitzman, S. & Gordon, L. (1990) Blood 76, 655-663. [PubMed] [Google Scholar]
- 9.Adam, L., Fabian, I., Suzuki, K. & Gordon, G. (1992) Inorg. Chem. 31, 3534-3541. [Google Scholar]
- 10.White, G. C. (1972) in Handbook of Chlorination (Van Nostrand Reinhold, New York), pp. 182-227.
- 11.Hazen, S. L., Hsu, F. F., Mueller, D. M., Crowley, J. R. & Heinecke, J. W. (1996) J. Clin. Invest. 98, 1283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazen, S. L., Hsu, F. F., Duffin, K. & Heinecke, J. W. (1996) J. Biol. Chem. 271, 23080-23088. [DOI] [PubMed] [Google Scholar]
- 13.Henderson, J. P., Byun, J. & Heinecke, J. W. (1999) J. Biol. Chem. 274, 33440-33448. [DOI] [PubMed] [Google Scholar]
- 14.Prutz, W. A. (1996) Arch. Biochem. Biophys. 332, 110-120. [DOI] [PubMed] [Google Scholar]
- 15.Drozdz, R., Naskalski, J. W. & Sznajd, J. (1988) Biochim. Biophys. Acta 957, 47-52. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins, C. L., Pattison, D. I. & Davies, M. J. (2003) Amino Acids 25, 259-274. [DOI] [PubMed] [Google Scholar]
- 17.Masuda, M., Suzuki, T., Friesen, M. D., Ravanat, J.-L., Cadet, J., Pignatelli, B., Nishino, H. & Ohshima, H. (2001) J. Biol. Chem. 276, 40486-40496. [DOI] [PubMed] [Google Scholar]
- 18.Domingan, N. M., Charlton, T. S., Duncan, M. W., Winterbourn, C. C. & Kettle, A. J. (1995) J. Biol. Chem. 270, 16542-16548. [DOI] [PubMed] [Google Scholar]
- 19.Hazen, S. L. & Heinecke, J. W. (1997) J. Clin. Invest. 99, 2075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kettle, A. J. (1996) FEBS Lett. 379, 103-106. [DOI] [PubMed] [Google Scholar]
- 21.Winterbourn, C. C., van den Berg, J. J., Roitman, E. & Kuypers, F. A. (1992) Arch. Biochem. Biophys. 296, 547-555. [DOI] [PubMed] [Google Scholar]
- 22.Carr, A. C., van den Berg, J. J. M. & Winterbourn, C. C. (1996) Arch. Biochem. Biophys. 332, 63-69. [DOI] [PubMed] [Google Scholar]
- 23.Panasenko, O. M. (1997) Biofactors 6, 181-190. [DOI] [PubMed] [Google Scholar]
- 24.Spickett, C. M., Jerlich, A., Panasenko, O. M., Arnhold, J., Pitt, A. R., Stelmaszynska, T. & Schaur, R. J. (2000) Acta Biochim. Pol. 47, 889-899. [PubMed] [Google Scholar]
- 25.Panasenko, O. M., Evgina, S. A., Aidyraliev, R. K., Sergienko, V. I. & Vladimirov, Y. A. (1994) Free Radical Biol. Med. 16, 143-148. [DOI] [PubMed] [Google Scholar]
- 26.Panasenko, O. M., Arnhold, J., Vladimirov Yu, A., Arnhold, K. & Sergienko, V. I. (1997) Free Radical Res. 27, 1-12. [DOI] [PubMed] [Google Scholar]
- 27.Panasenko, O. M. & Arnhold, J. (1999) Free Radical Res. 30, 479-487. [DOI] [PubMed] [Google Scholar]
- 28.Khan, A. U. & Kasha, M. (1970) J. Am. Chem. Soc. 92, 3293-3300. [Google Scholar]
- 29.Held, A. M., Halko, D. J. & Hurst, J. K. (1978) J. Am. Chem. Soc. 100, 5732-5740. [Google Scholar]
- 30.Murray, R. W. (1979) in Singlet Oxygen, eds. Wasserman, H. H. & Murray, R. W. (Academic, New York), pp. 59-114.
- 31.Girotti, A. W. (1998) J. Lipid Res. 39, 1529-1542. [PubMed] [Google Scholar]
- 32.Hasty, N., Merkel, P. B., Radlick, P. & Kearns, D. R. (1972) Tetrahedron Lett. 49-52.
- 33.Merkel, P. & Kearns, D. (1972) J. Am. Chem. Soc. 94, 1029-1030. [Google Scholar]
- 34.Kajiwara, T. & Kearns, D. R. (1973) J. Am. Chem. Soc. 95, 5886-5890. [Google Scholar]
- 35.Martinez, G. R., Ravanat, J. L., Cadet, J., Miyamoto, S., Medeiros, M. H. & Di Mascio, P. (2004) J. Am. Chem. Soc. 126, 3056-3057. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto, S., Martinez, G. R., Medeiros, M. H. & Di Mascio, P. (2003) J. Am. Chem. Soc. 125, 6172-6179. [DOI] [PubMed] [Google Scholar]
- 37.Russell, G. A. (1957) J. Am. Chem. Soc. 79, 3871-3877. [Google Scholar]
- 38.Bersohn, M. & Thomas, J. R. (1964) J. Am. Chem. Soc. 86, 959. [Google Scholar]
- 39.Ingold, K. U. (1969) Accts. Chem. Res. 2, 1-9. [Google Scholar]
- 40.Kalyanaraman, B., Mottley, C. & Mason, R. P. (1983) J. Biol. Chem. 258, 3855-3858. [PubMed] [Google Scholar]
- 41.Chamulitrat, W. & Mason, R. P. (1989) J. Biol. Chem. 264, 20968-20973. [PubMed] [Google Scholar]
- 42.Howard, J. A. & Ingold, K. U. (1968) J. Am. Chem. Soc. 90, 1056-1058. [Google Scholar]
- 43.Niu, Q. & Mendenhall, G. (1992) J. Am. Chem. Soc. 114, 165-172. [Google Scholar]
- 44.Chan, H. W.-S., Levett, G. & Matthew, J. A. (1979) Chem. Phys. Lipids 24, 245-256. [Google Scholar]
- 45.Cahill, A. E. & Taube, H. (1952) J. Am. Chem. Soc. 74, 2312-2318. [Google Scholar]
- 46.Koppenol, W. H. (1994) FEBS Lett. 347, 5-8. [DOI] [PubMed] [Google Scholar]
- 47.Koppenol, W. H. (1990) FEBS Lett. 264, 165-167. [DOI] [PubMed] [Google Scholar]
- 48.Buettner, G. R. (1993) Arch. Biochem. Biophys. 300, 535-543. [DOI] [PubMed] [Google Scholar]
- 49.Johnson, D. W. & Margerum, D. W. (1991) Inorg. Chem. 30, 4845-4851. [Google Scholar]
- 50.Eiserich, J. P., Cross, C. E., Jones, A. D., Halliwell, B. & van der Vliet, A. (1996) J. Biol. Chem. 271, 19199-19208. [DOI] [PubMed] [Google Scholar]
- 51.Davies, M. J. & Hawkins, C. L. (2000) Free Radical Res. 33, 719-729. [DOI] [PubMed] [Google Scholar]
- 52.Hawkins, C. L. & Davies, M. J. (1998) Biochem. J. 332, 617-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawkins, C. L. & Davies, M. J. (1998) J. Chem. Soc. Perkin Trans. 2, 1937-1945. [Google Scholar]
- 54.Wardman, P. (1989) J. Phys. Chem. Ref. Data 18, 1637-1755. [Google Scholar]
- 55.Long, C. A. & Bielski, B. H. J. (1988) J. Phys. Chem. 84, 555-557. [Google Scholar]
- 56.Folkes, L. K., Candeias, L. P. & Wardman, P. (1995) Arch. Biochem. Biophys. 323, 120-126. [DOI] [PubMed] [Google Scholar]
- 57.Candeias, L. P., Patel, K. B., Stratford, M. R. L. & Wardman, P. (1993) FEBS Lett. 333, 151-153. [DOI] [PubMed] [Google Scholar]
- 58.Koppenol, W. H., Moreno, J. J., Pryor, W. A., Ischiropoulos, H. & Beckman, J. S. (1992) Chem. Res. Toxicol. 5, 834-842. [DOI] [PubMed] [Google Scholar]
- 59.Cadet, J. & Di Mascio, P. (2006) in The Chemistry of Peroxides, ed. Rappaport, Z. (Wiley, Chichester, U.K.), Vol. 2.
- 60.Klotz, L. O., Kroncke, K. D. & Sies, H. (2003) Photochem. Photobiol. Sci. 2, 88-94. [DOI] [PubMed] [Google Scholar]
- 61.Podrez, E. A., Abu-Soud, H. M. & Hazen, S. L. (2000) Free Radical Biol. Med. 28, 1717-1725. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, R., Brennan, M.-L., Shen, Z., MacPherson, J. C., Schmitt, D., Molenda, C. E. & Hazen, S. L. (2002) J. Biol. Chem. 277, 46116-46122. [DOI] [PubMed] [Google Scholar]
- 63.Morris, J. C. (1966) J. Phys. Chem. 70, 3798-3805. [Google Scholar]
- 64.Miyamoto, S., Dupas, C., Murota, K. & Terao, J. (2003) Lipids 38, 641-649. [DOI] [PubMed] [Google Scholar]
- 65.Di Mascio, P. & Sies, H. (1989) J. Am. Chem. Soc. 111, 2909-2914. [Google Scholar]
- 66.Martinez, G. R., Di Mascio, P., Bonini, M. G., Augusto, O., Briviba, K., Sies, H., Maurer, P., Rothlisberger, U., Herold, S. & Koppenol, W. H. (2000) Proc. Natl. Acad. Sci. USA 97, 10307-10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyamoto, S., Martinez, G. R., Martins, A. P., Medeiros, M. H. & Di Mascio, P. (2003) J. Am. Chem. Soc. 125, 4510-4517. [DOI] [PubMed] [Google Scholar]
- 68.Bonini, M. G., Radi, R., Ferrer-Sueta, G., Ferreira, A. M. D. C. & Augusto, O. (1999) J. Biol. Chem. 274, 10802-10806. [DOI] [PubMed] [Google Scholar]
- 69.Mehlhorn, R. J. & Keith, D. A. (1972) in Membrane Molecular Biology, eds. Fox, C. F. & Keith, D. A. (Sinauer, Stanford, CT), pp. 192-227.
- 70.Duling, D. R. (1994) J. Magn. Reson. 104, 105-110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.