Abstract
The association between Mtr2 and Mex67 is essential for the nuclear export of bulk messenger RNA in yeast. In metazoans, the analogous function is carried out by the TAP–p15 heterodimer. Whereas Mex67 and TAP are highly conserved proteins, their binding partners, Mtr2 and p15, share no sequence similarity, but are nevertheless functionally homologous. Here, we report the 2.8-Å resolution crystal structure of Mtr2 in complex with the NTF2-like domain of Mex67. Mtr2 is a novel member of the NTF2-like family and interacts with Mex67, forming a complex with a similar structural architecture to that of TAP–p15. Mtr2 fulfils an analogous function to that of human p15 in maintaining the structural integrity of the heterodimer. In addition, Mtr2 presents a long internal loop, which contains residues that affect the export of the large ribosomal subunit.
Introduction
Export of messenger RNAs from the nucleus to the cytoplasm in eukaryotic cells occurs through nuclear pore complexes (NPCs) and is mediated by transport factors that are able to interact both with components of NPCs and with mRNA export cargoes (for a review, see Izaurralde, 2002; Reed & Hurt, 2002). The yeast Mex67–Mtr2 heterodimer and the metazoan TAP–p15 heterodimer are important mRNA nuclear export factors. Inactivation or depletion of these proteins in Saccharomyces cerevisiae, Caenorhabditis elegans and Drosophila melanogaster results in a rapid and strong poly(A)+ RNA accumulation in the nucleoplasm, and is eventually lethal (Herold et al., 2001; Segref et al., 1997; Tan et al., 2000). The function of the Mex67–Mtr2 heterodimer is not only essential, but is also evolutionary conserved to such an extent that the lethal phenotype of its knockout can be partially rescued by co-expression in yeast of human TAP and p15 (Katahira et al., 1999).
The larger subunit of the heterodimer (Mex67/TAP) belongs to the conserved family of nuclear export factor (NXF) proteins (Herold et al., 2001). Structural studies of human TAP have shown a modular architecture, with four globular domains linked by flexible regions (for a review, see Conti & Izaurralde, 2001). The amino-terminal half recognizes mRNA export cargoes and includes a RNA-binding domain (RBD) and a leucine-rich repeat (LRR) domain (Braun et al., 1999; Liker et al., 2000). The carboxy-terminal half docks to the NPC and consists of a NTF2-like domain and a UBA-like domain (Bachi et al., 2000; Fribourg et al., 2001; Grant et al., 2003). Mex67 and metazoan TAP share at least 22% sequence identity in the LRR, NTF2-like and UBA-like regions, which are likely to have similar functions in the context of mRNA export.
The NPC-binding mechanism of human TAP has been dissected at the molecular level. Both of the C-terminal domains of TAP bind directly to the Phe-Gly (FG) repeats that characterize the proteins of the NPC (known as nucleoporins) and function synergistically for efficient NPC translocation (Fribourg et al., 2001; Grant et al., 2003). To be functional in FG–nucleoporin binding, the NTF2-like domain of TAP has to form a heterodimer with another NTF2-like molecule, p15. Although p15 does not bind nucleoporins directly, the function of this subunit is essential for maintaining the NTF2-like domain of TAP in a correctly folded conformation for FG binding, thus enhancing the ability of TAP to bind to nucleoporins (Braun et al., 2002; Fribourg et al., 2001; Wiegand et al., 2002). The functional counterpart of p15 in yeast is Mtr2, which binds the NTF2-like domain of Mex67 and is required for interaction with nucleoporins, either directly or indirectly (Santos-Rosa et al., 1998; Strasser et al., 2000; Strawn et al., 2001). However, Mtr2 has no detectable sequence similarity to p15.
No Mtr2 homologue is present in the human genome and no p15 homologue is present in the yeast genome. Mtr2 and human p15 seem to be functional homologues that recognize Mex67 and TAP in a species-dependent manner (Katahira et al., 1999). The question arises as to whether they have similar structural features to allow them to carry out a similar function in mRNA export. As Mtr2 has also been implicated in the export of the large ribosomal subunit in yeast (Stage-Zimmermann et al., 2000; Bassler et al., 2001), the question also arises as to the nature of the structural differences that are responsible for this distinct and separable function. To address these questions, we have determined the structure of Mtr2 in complex with the interacting domain of Mex67.
Results and Discussion
Mtr2 and Mex67 interact to form a NTF2-like heterodimer
The interaction between yeast Mtr2 and Mex67 occurs through the formation of a NTF2-like heterodimer (Fig. 1). The NTF2-like structure of each protein consists of a curved βsheet, with a long N-terminal helix and a short helix filling its concave side. The two proteins heterodimerize using the convex faces of their β-sheets. The structures of the two subunits of the heterodimer are similar to each other and to other NTF2-like proteins (Fig. 1), with at least 50% of the residues superposing with a root mean square deviation (r.m.s.d.) of less than 2.5 Å. Superposition of Mex67 and TAP (Fig. 2A) results in an r.m.s.d. of 2.5 Å for 130 Cα atoms, whereas superposition of Mtr2 and p15 (Fig. 2B) has a r.m.s.d. of 2.4 Å for 117 residues, as compared with an r.m.s.d. of 2.5 Å for the superposition of 118 residues of Mex67 with TAP. Similarly to TAP, Mex67 has an extra βstrand and insertion region that are not present in Mtr2, p15 or NTF2 (Fig. 3). The extra strand (β7) in Mex67 is at the equivalent structural position to strand β0 in TAP (Fig. 2A; Fribourg et al., 2001). The insertion region that contributes to heterodimerization in TAP is disordered in the Mex67 structure (residues 316–353; shaded in grey in Fig. 3).
Figure 1.
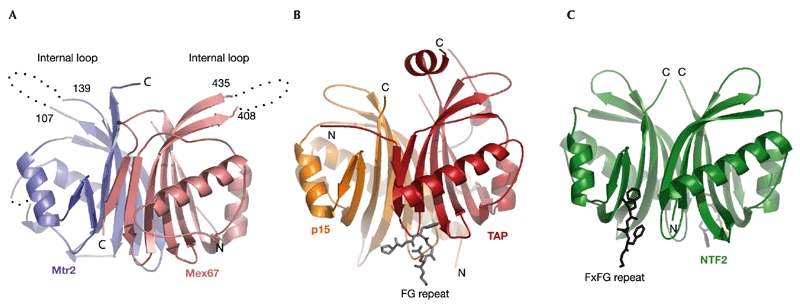
Mtr2 and Mex67 interact to form an NTF2-like heterodimer similar to human TAP–p15 and NTF2. The structures of Mex67–Mtr2 (A), TAP–p15 (B) and NTF2–NTF2 (C) complexes are shown in similar orientations after optimal superposition. The location of the Phe-Gly (FG)-binding sites are shown for TAP (Fribourg et al., 2001) and NTF2 (Bayliss et al., 2002). Portions of the molecules not present in the crystallographic models are shown with dots.
Figure 2.
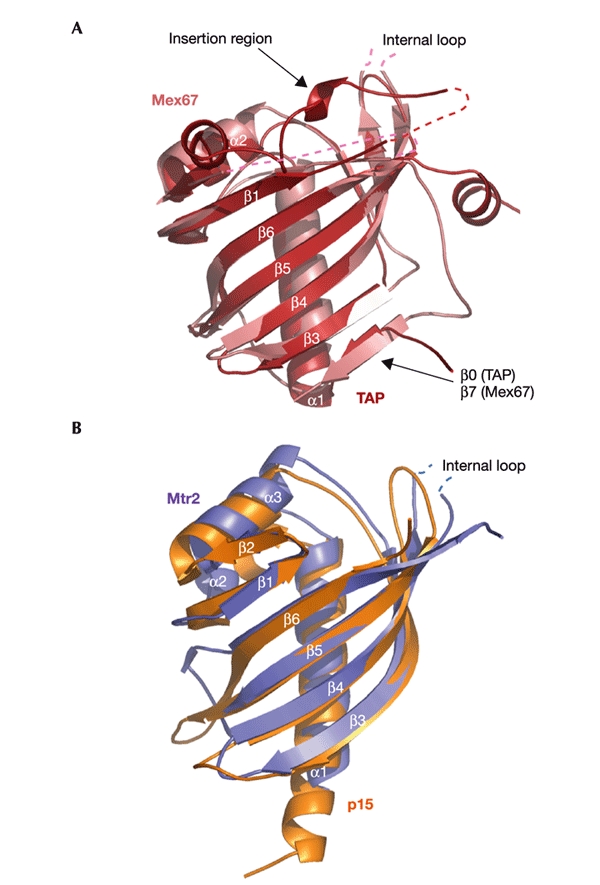
Structural similarity of the NTF2-like domains of the yeast and human messenger RNA export factors. The structures of Mex67 and TAP (A) and of Mtr2 and p15 (B) are shown superposed and in the same relative orientation. The secondary-structure elements and the position of the internal loops of Mex67 and Mtr2 are labelled. The insertion region of TAP is indicated.
Figure 3.
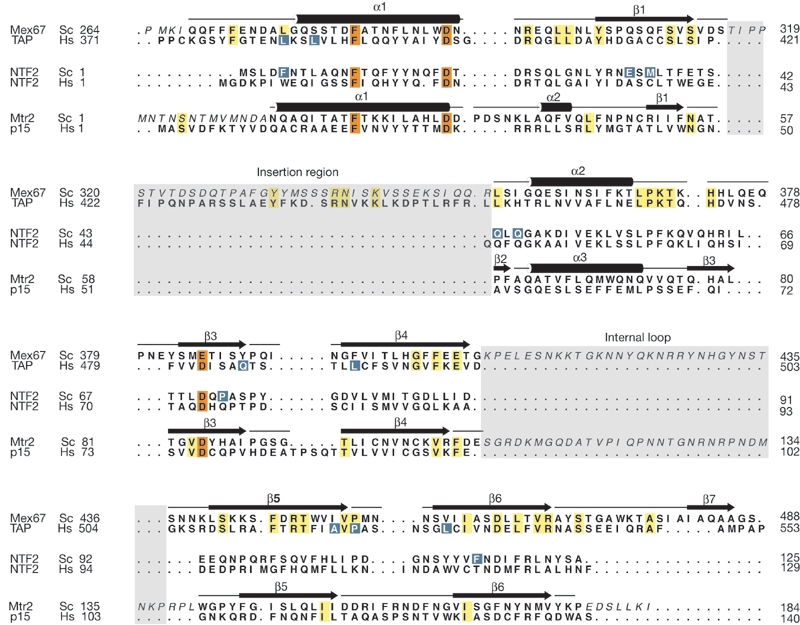
Structure-based sequence alignment of NTF2-like domains involved in nuclear transport. Sequence conservation is shown by colour coding. Invariant residues shared by Mex67 and TAP or shared by Mtr2 and p15 are highlighted in yellow. Residues that are conserved among NTF2-like folds are highlighted in orange. Secondary-structure elements of Mex67 and Mtr2 are indicated. The insertion region characteristic of nuclear export factor (NXF) proteins (Mex67 and TAP) is shaded in grey. The long internal loop present in the yeast Mex67 and Mtr2 proteins is also shaded in grey. Residues for which no ordered electron density is present are shown in italics. Residues contributing to Phe-Gly (FG) nucleoporin-binding sites (see also Fig. 4) are shown in blue.
In the case of Mtr2, the homology with NTF2-like molecules is remarkable in light of the negligible sequence similarity (sequence identity of <9%). The few residues that are conserved at equivalent positions (highlighted in orange in Fig. 3) have a structural role and are likely to be the hallmarks of this fold. In particular, the interaction between an aspartic acid at the end of the N-terminal helix (Asp 31) and a histidine from a central βstrand (His 78) is well conserved. A similar interaction is present in Mex67 (Asp 293 and His 373), human TAP (Asp 399 and His 474) and NTF2 (Asp 21 and His 64; Fig. 3). The interaction is also conserved in NTF2-like proteins that are not involved in nuclear transport, such as scytalone dehydratase (Bullock et al., 1996; Lundqvist et al., 1994). The N-terminal α-helix is also anchored to the βsheet by hydrophobic interactions that involve a conserved phenylalanine residue (Phe 22 in Mtr2; Fig. 3).
In comparison with other NTF2-like molecules, both Mtr2 and Mex67 have an extra internal loop. The 30-residue loop was predicted in Mex67 on the basis of sequence alignment with TAP (Fig. 3) and was deleted in the protein that was used to obtain crystals. Remarkably, the crystallographic analysis shows that Mtr2 has a 35-residue loop at the same position, between strands β4 and β5 of the NTF2-like fold (shaded in grey in Fig. 3). No ordered electron density is present for most of the loop (Figs 1,2).
Species-specific interaction between Mtr2 and Mex67
The heterodimerization interface between Mtr2 and Mex67 shows similar interactions to those seen in the human TAP–p15 structure. Relatively subtle differences might therefore be responsible for the species-specific recognition of Mtr2 and p15 by their respective NXF orthologues (Katahira et al., 1999). These include sterically compatible interactions between small and large polar side-chains. In the case of the yeast complex, the small Ser 148 side-chain of Mtr2 interacts with Asp 446 of Mex67. At the corresponding structural position in the human complex, Asn 110 of p15 interacts with the small Thr 514 side-chain of TAP (Fig. 3). Furthermore, the adjacent patch of residues has polar features in the case of the yeast complex (Asn 99 of Mtr2 with Thr 466) and hydrophobic interactions occur at the corresponding structural positions of the human complex (Cys 96 of p15 with Phe 535 and the nearby Pro 421 of TAP; Fig. 3).
An acidic residue that has a crucial function in heterodimerization in the human complex (Asp 482 of TAP and Asp 76 in p15; Fribourg et al., 2001) is present at a conserved structural position in the yeast complex (Fig. 3). In Mex67, the corresponding residue, Glu 385, is involved in an intermolecular interaction with Asn 170 of Mtr2 and in an intramolecular interaction with His 400. The mutation of His 400 to Tyr leads to the thermosensitive mex67-5 allele (Segref et al., 1997). This mutation impairs complex formation and results in mislocalization of the protein to the cytoplasm when the cells are shifted to a restrictive temperature.
Functional implications
Binding of FG-repeat nucleoporins to the NTF2-like domain of TAP occurs at a single hydrophobic pocket (consisting of Pro 521, Leu 386 and Leu 491; Fig. 4A). The equivalent position in Mex67 is lined by hydrophobic residues, but larger side-chains (Phe 272 and Phe 395 of Mex67 rather than Leu 386 and Leu 491 of TAP) and a different conformation in a nearby loop effectively occlude a potential pocket. However, the different conformation (Pro 453 of Mex67 compared with the position observed for Pro 521 of TAP; Fig. 4A,B) is probably the result of crystal contacts with a neighbouring molecule. It is therefore possible that this site is accessible for nucleoporin binding in solution. In the case of Mtr2, this site is more hydrophilic (with Asp 155 at the same structural position as Pro 521 of TAP; Fig 4C). An FG-binding site at the location corresponding to the site in the NTF2 N77Y structure (Bayliss et al., 2002; Fig. 4D) is unlikely to be present in the Mex67–Mtr2 complex, due to the presence of the additional βstrand and insertion region occupying this site.
Figure 4.
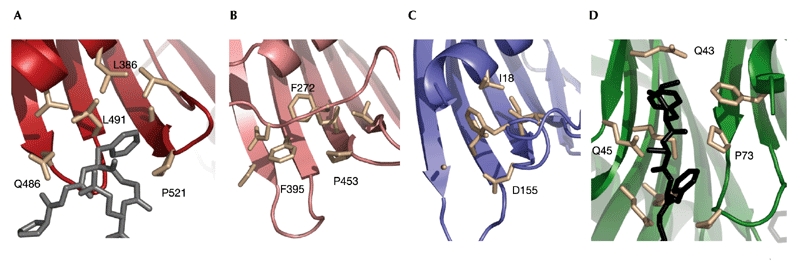
Comparison of nucleoporin-binding abilities of TAP, Mex67, Mtr2 and NTF2. The structural region corresponding to the Phe-Gly (FG)-binding site of TAP (A) is shown for Mex67 (B) and Mtr2 (C). The FxFG binding pocket of yeast NTF2 N77Y is shown in (D).
Most mutant alleles of MTR2 are impaired in nuclear mRNA export. The mtr2-190 and mtr2-142 alleles are synthetically lethal when combined with mutations of other genes involved in mRNA export (mex67-5 and sac3Δ139). They carry a mutation in a similar structural region (L140P and G142D; Lei et al., 2003; Santos-Rosa et al., 1998). The mutated residues are located at the end of the 35-residue internal loop of Mtr2 (Fig. 3). A double mutation at the beginning of the same loop (E106G and R109G) produces the mtr2-33 allele, which does not show mRNA export defects, but instead shows defective export of the 60S ribosomal subunit in yeast (Bassler et al., 2001). Remarkably, the long internal loop of Mtr2 and the corresponding internal loop of Mex67 would face each other at the open crevice of the NTF2-like heterodimer (Fig. 1). These loops are the main structural difference between the yeast and human complexes. In conclusion, the NTF2-like domains of Mex67–Mtr2 and TAP–p15 are functionally and structurally conserved through evolution. Nevertheless, localized structural differences at the long loop regions of the yeast proteins might provide additional species-dependent functionality.
Methods
Protein production and crystallization.
S. cerevisiae full-length Mtr2 and a Mex67 construct comprising residues 264–488 (where an internal loop comprising residues 408–435 had been deleted by mutagenesis) were cloned into a pET-28c vector (Novagen) and a pGEX vector (Amersham), respectively, and co-expressed in Escherichia coli. The complex was purified by affinity chromatography using a glutathione-S-transferase tag on Mex67. After cleavage of the tag by TEV protease, an extra anion-exchange purification step was used to obtain a homogeneous complex preparation. Needle-like crystals were grown at 18 °C by vapour diffusion in sitting drops by mixing equal volumes of the well solution containing 20% polyethylene glycol (PEG) 3000, 200 mM calcium acetate, 100 mM Hepes, pH 7.5, and protein solution at 10 mg ml−1. The crystals were cryoprotected with 15% glycerol and diffracted to a 2.8-Å resolution using synchrotron radiation. No crystals could be obtained from a similarly purified complex where the internal loop of Mex67 had not been deleted.
Structure determination.
Phases were obtained at a 3.0-Å resolution using the SOLVE/RESOLVE package from SAD data recorded on a Se-methionine-substituted protein crystal combined with data from a mercury derivative (Terwilliger, 2000; Terwilliger & Berendzen, 1999). The model was built in O and refined to an Rfree of 26.2% at 2.8-Å resolution against the best data set (collected on a poorly substituted mercury derivative crystal) with CNS (Brunger et al., 1998; Jones et al., 1991). The final model comprises residues 14–106 and 141–177 of Mtr2 and residues 268–315 and 354–488 of Mex67. Continuous electron density is present between residues Gly 407 and Ser 436 in the engineered β4–β5 loop. Data collection and refinement statistics are shown in Table 1. The atomic coordinates and structure factors have been deposited in Protein Data Bank (accession code ).
Table 1.
Data collection, phasing and refinement statistics
| Native | Se-Met Peak | EMP derivative | |
|---|---|---|---|
| Data collection and phasing statistics | |||
| Space group | P212121 | – | – |
| Cell dimensions (Å) | 48.81, 83.71, 85.52 | 48.88, 82.62, 84.75 | 48.67, 82.48, 84.85 |
| X-ray source | ESRF ID14-1 | ESRF ID14-4 | ESRF ID14-1 |
| Wavelength (Å) | 0.934 | 0.9792 | 0.934 |
| Resolution (Å) | 30–2.8 (2.8–2.95) | 30–3.25 (3.25–3.47) | 30–2.9 (2.9–3.06) |
| Unique reflections | 9,718 | 6,951 | 7,942 |
| Redundancy | 3.7 (2.7) | 3.4 (3.5) | 3.7 (2.8) |
| Completeness (%) | 95.6 (78.5) | 95.6 (95.3) | 99.2 (96.5) |
| I/σ | 9.4 (2.0) | 4.8 (6.6) | 9.3 (2.5) |
| Rsym (%) | 6.9 (37.0) | 6.7 (11.5) | 6.8 (30.5) |
| FOM (acentric) | - | 0.45 | – |
| Refinement statistics | |||
| Rfree (%) | 26.2 | – | – |
| Rworking (%) | 24.2 | – | – |
| φψ most favoured (%) | 81.5 | – | – |
| φψ additionally allowed (%) | 18.5 | – | – |
| Protein residues | 285 | – | – |
| Water molecules | 3 | – | – |
| Ions | 1 Hg | – | – |
Values for the outermost resolution shell are shown in brackets. EMP, ethyl mercuric phosphate; FOM, figure of merit; Rfree, R factor of 5% of the reflections excluded from the refinement.
Acknowledgments
We thank the staff at the ESRF, SLS and DESY synchrotrons for assistance during data collection, in particular R. Ravelli at ID14-4. We acknowledge E. Izaurralde for the expression clones. We also thank P. Brick and E. Izaurralde for critical reading of the manuscript. S.F. is supported by a Marie Curie fellowship (HPMF-2000-1018).
References
- Bachi A. et al. (2000) The C-terminal domain of TAP interacts with the nuclear pore complex and promotes export of specific CTE-bearing RNA substrates. RNA, 6, 136–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler J., Grandi P., Gadal O., Lessmann T., Petfalski E., Tollervey D., Lechner J. & Hurt E. (2001) Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol. Cell, 8, 517–529. [DOI] [PubMed] [Google Scholar]
- Bayliss R., Leung S.W., Baker R.P., Quimby B.B., Corbett A.H. & Stewart M. (2002) Structural basis for the interaction between NTF2 and nucleoporin FxFG repeats. EMBO J., 21, 2843–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I.C., Rohrbach E., Schmitt C. & Izaurralde E. (1999) TAP binds to the constitutive transport element (CTE) through a novel RNA-binding motif that is sufficient to promote CTE-dependent RNA export from the nucleus. EMBO J., 18, 1953–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I.C., Herold A., Rode M. & Izaurralde E. (2002) Nuclear export of mRNA by TAP/NXF1 requires two nucleoporin-binding sites but not p15. Mol. Cell. Biol., 22, 5405–5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. [DOI] [PubMed] [Google Scholar]
- Bullock T.L., Clarkson W.D., Kent H.M. & Stewart M. (1996) The 1.6 Å resolution crystal structure of nuclear transport factor 2 (NTF2). J. Mol. Biol., 260, 422–431. [DOI] [PubMed] [Google Scholar]
- Conti E. & Izaurralde E. (2001) Nucleocytoplasmic transport enters the atomic age. Curr. Opin. Cell Biol., 13, 310–319. [DOI] [PubMed] [Google Scholar]
- Fribourg S., Braun I.C., Izaurralde E. & Conti E. (2001) Structural basis for the recognition of a nucleoporin FG repeat by the NTF2-like domain of the TAP/p15 mRNA nuclear export factor. Mol. Cell, 8, 645–656. [DOI] [PubMed] [Google Scholar]
- Grant R.P., Neuhaus D. & Stewart M. (2003) Structural basis for the interaction beetween the Tap/NXF1 UBA domain and FG nucleoporins at 1Å resolution. J. Mol. Biol., 326, 849–858. [DOI] [PubMed] [Google Scholar]
- Herold A., Klymenko T. & Izaurralde E. (2001) NXF1/p15 heterodimers are essential for mRNA nuclear export in Drosophila. RNA, 7, 1768–1780. [PMC free article] [PubMed] [Google Scholar]
- Izaurralde E. (2002) A novel family of nuclear transport receptors mediates the export of messenger RNA to the cytoplasm. Eur. J. Cell Biol., 81, 577–584. [DOI] [PubMed] [Google Scholar]
- Jones T.A., Zou J.Y., Cowan S.W. & Kjeldgaard M. (1991) Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A, 47, 110–119. [DOI] [PubMed] [Google Scholar]
- Katahira J., Strasser K., Podtelejnikov A., Mann M., Jung J.U. & Hurt E. (1999) The Mex67p-mediated nuclear mRNA export pathway is conserved from yeast to human. EMBO J., 18, 2593–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei E.P., Stern C.A., Fahrenkrog B., Krebber H., Moy T.I., Aebi U. & Silver P.A. (2003) Sac3 is an mRNA export factor that localizes to cytoplasmic fibrils of nuclear pore complex. Mol. Biol. Cell, 14, 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liker E., Fernandez E., Izaurralde E. & Conti E. (2000) The structure of the mRNA export factor TAP reveals a cis arrangement of a non-canonical domain and a LRR domain. EMBO J., 19, 5587–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist T., Rice J., Hodge C.N., Basarab G.S., Pierce J. & Lindqvist Y. (1994) Crystal structure of scytalone dehydratase—a disease determinant of the rice pathogen, Magnaporthe grisea. Structure, 2, 937–944. [DOI] [PubMed] [Google Scholar]
- Reed R. & Hurt E. (2002) A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell, 108, 523–531. [DOI] [PubMed] [Google Scholar]
- Santos-Rosa H., Moreno H., Simos G., Segref A., Fahrenkrog B., Pante N. & Hurt E. (1998) Nuclear mRNA export requires complex formation between Mex67p and Mtr2p at the nuclear pores. Mol. Cell. Biol., 18, 6826–6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segref A., Sharma K., Doye V., Hellwig A., Huber J., Luhrmann R. & Hurt E. (1997) Mex67p, a novel factor for nuclear mRNA export, binds to both poly(A)+ RNA and nuclear pores. EMBO J., 16, 3256–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stage-Zimmermann T., Schmidt U. & Silver P.A. (2000) Factors affecting nuclear export of the 60S ribosomal subunit in vivo. Mol. Biol. Cell, 11, 3777–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser K., Bassler J. & Hurt E. (2000) Binding of the Mex67p/Mtr2p heterodimer to FXFG, GLFG, and FG repeat nucleoporins is essential for nuclear mRNA export. J. Cell Biol., 150, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawn L.A., Shen T. & Wente S.R. (2001) The GLFG regions of Nup116p and Nup100p serve as binding sites for both Kap95p and Mex67p at the nuclear pore complex. J. Biol. Chem., 276, 6445–6452. [DOI] [PubMed] [Google Scholar]
- Tan W., Zolotukhin A.S., Bear J., Patenaude D.J. & Felber B.K. (2000) The mRNA export in Caenorhabditis elegans is mediated by Ce-NXF-1, an ortholog of human TAP/NXF and Saccharomyces cerevisiae Mex67p. RNA, 6, 1662–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger T.C. (2000) Maximum-likelihood density modification. Acta Crystallogr. D, 56, 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger T.C. & Berendzen J. (1999) Automated MAD and MIR structure solution. Acta Crystallogr. D, 55, 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand H.L., Coburn G.A., Zeng Y., Kang Y., Bogerd H.P. & Cullen B.R. (2002) Formation of Tap/NXT1 heterodimers activates Tap-dependent nuclear mRNA export by enhancing recruitment to nuclear pore complexes. Mol. Cell. Biol., 22, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


