Abstract
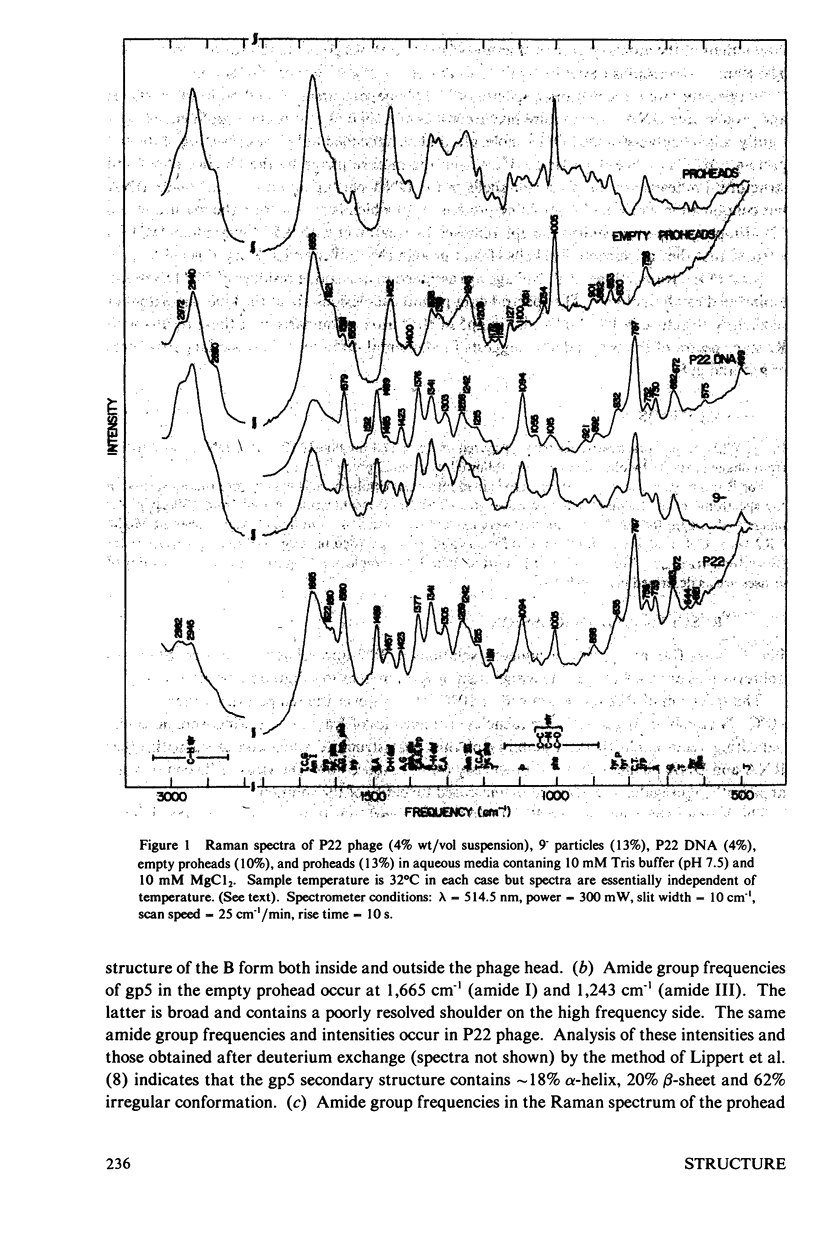
Laser Raman spectra of the DNA bacteriophage P22 and of its precursor particles and related structures have been obtained using 514.5-nm excitation. The spectra show that P22 DNA exists in the B form both inside of the phage head and after extraction from the phage. The major coat protein (gp5) contains a secondary structure composed of 18% α-helix, 20% β-sheet and 62% irregular conformations. The scaffolding protein (gp8) in the phage prohead is substantially richer than gp5 in α-helical content. Among the amino acid residues which give prominent Raman lines, the spectra show that tryptophans are exposed to solvent and most tyrosines are hydrogen bonded to positive donor groups. The above features of phage DNA and protein structures are nearly invariant to changes in temperature up to 80°C, indicating a remarkable thermal stability of the phage head and its encapsulated DNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Earnshaw W. C., Harrison S. C. DNA arrangement in isometric phage heads. Nature. 1977 Aug 18;268(5621):598–602. doi: 10.1038/268598a0. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Kiser E. J., Peticolas W. L. Determination of the backbone structure of nucleic acids and nucleic acid oligomers by laser Raman scattering. Proc Natl Acad Sci U S A. 1972 Apr;69(4):938–941. doi: 10.1073/pnas.69.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erfurth S. C., Peticolas W. L. Melting and premelting phenomenon in DNA by laser Raman scattering. Biopolymers. 1975 Feb;14(2):247–264. doi: 10.1002/bip.1975.360140202. [DOI] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Tyminski D., Desmeules P. J. Determination of the secondary structure of proteins by laser Raman spectroscopy. J Am Chem Soc. 1976 Oct 27;98(22):7075–7080. doi: 10.1021/ja00438a057. [DOI] [PubMed] [Google Scholar]
- Siamwiza M. N., Lord R. C., Chen M. C., Takamatsu T., Harada I., Matsuura H., Shimanouchi T. Interpretation of the doublet at 850 and 830 cm-1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds. Biochemistry. 1975 Nov 4;14(22):4870–4876. doi: 10.1021/bi00693a014. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., McDonald-Ordzie P. E., Hartman K. A. Studies of virus structure by laser-Raman spectroscopy. II. MS2 phage, MS2 capsids and MS2 RNA in aqueous solutions. J Mol Biol. 1976 Mar 25;102(1):103–124. doi: 10.1016/0022-2836(76)90076-0. [DOI] [PubMed] [Google Scholar]
- Thomas G., Jr, Murphy P. Structure of coat proteins in Pf1 and fd virions by laser raman spectroscopy. Science. 1975 Jun 20;188(4194):1205–1207. doi: 10.1126/science.1170637. [DOI] [PubMed] [Google Scholar]


