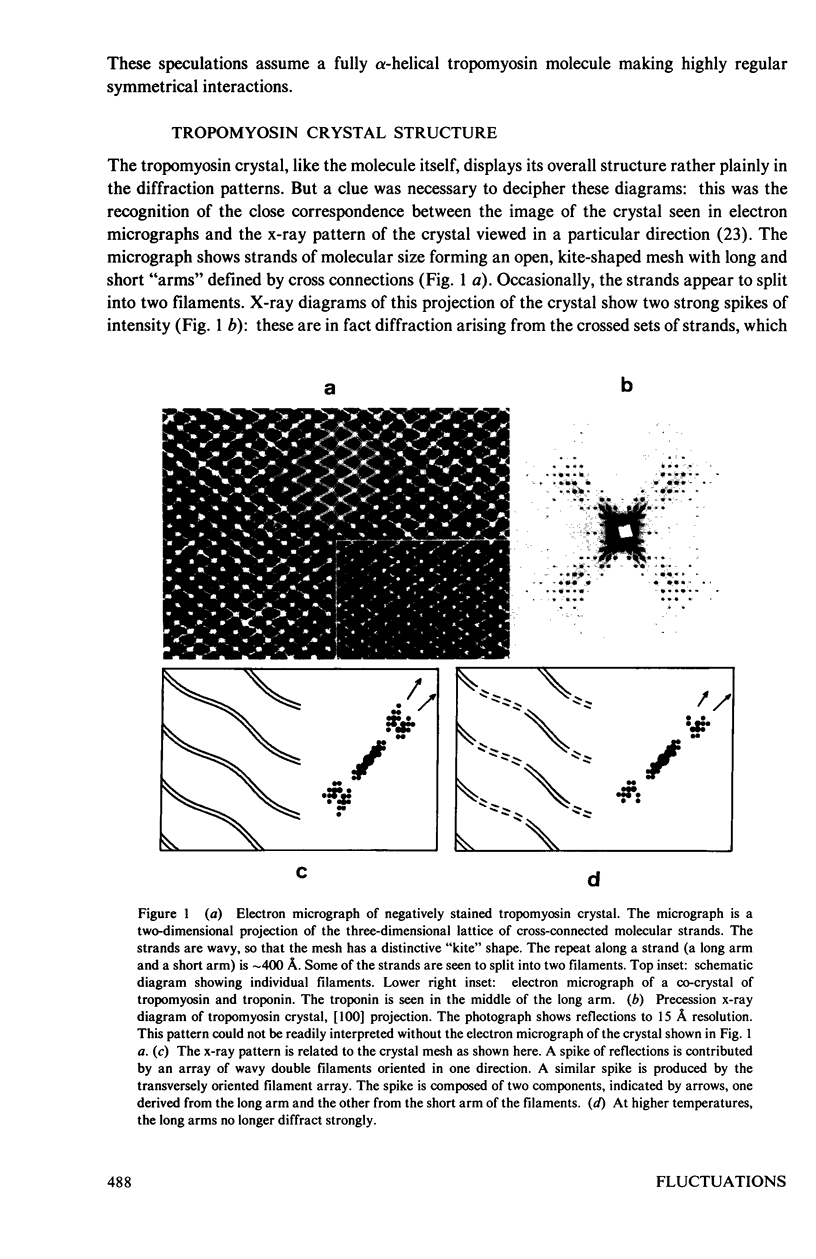
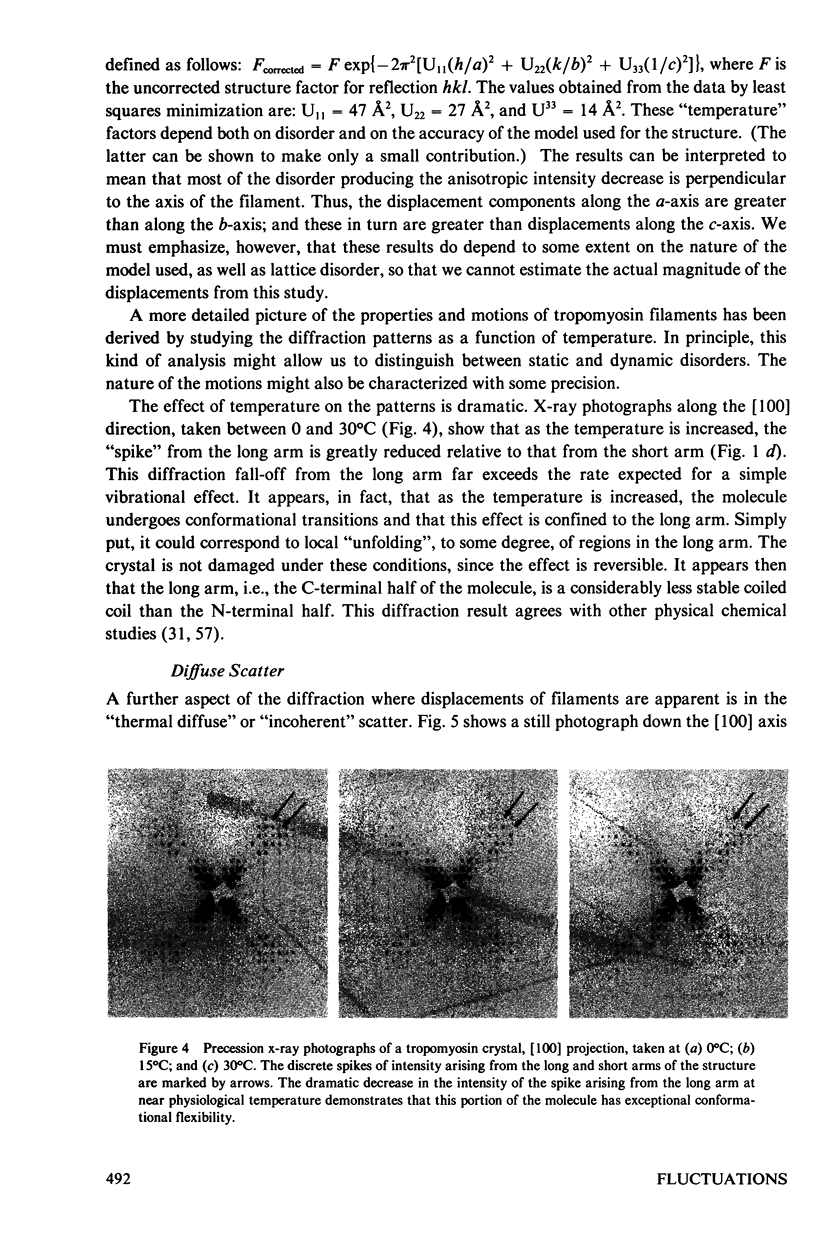
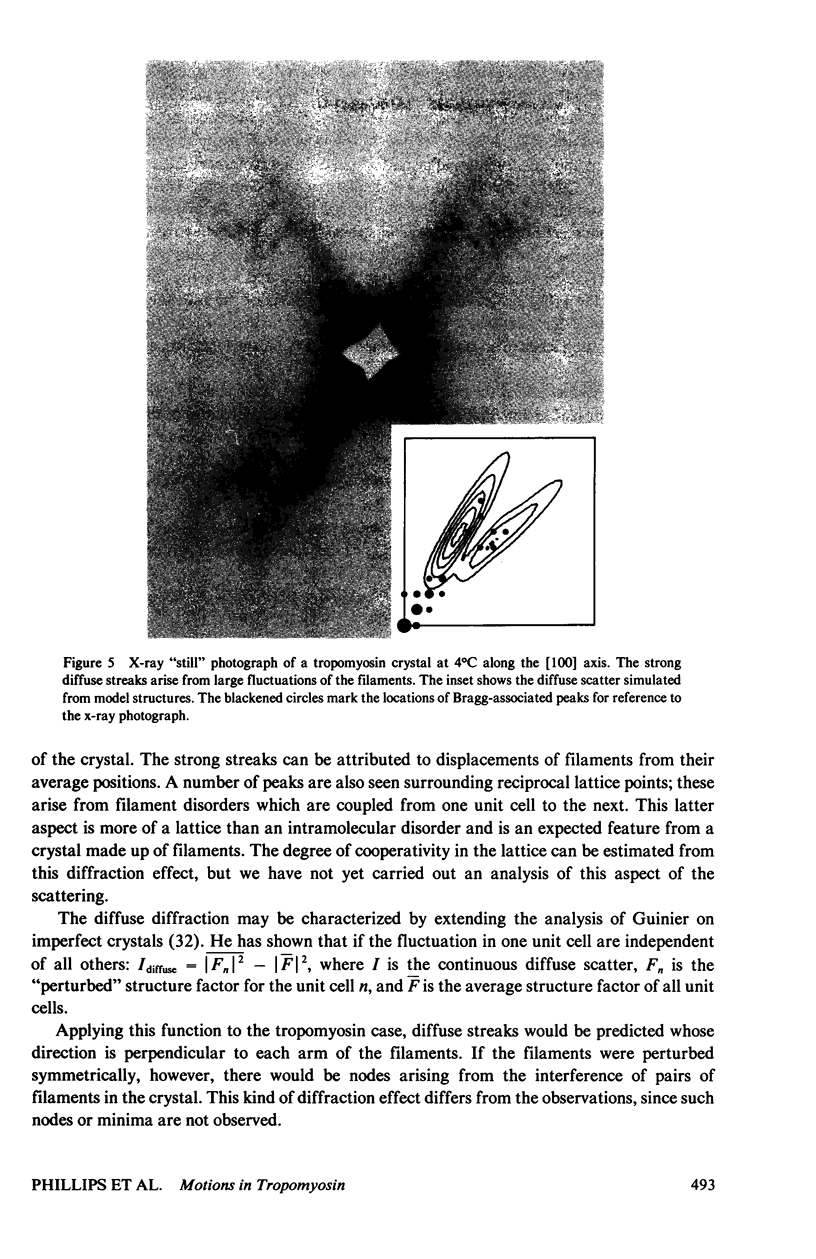
Abstract
Movements of tropomyosin play an essential role in muscle regulation. This fibrous protein is a two-chain alpha-helical coiled coil that bonds head to tail to form cables wound in the two long grooves of the actin helix. The regulatory switch consists of tropomyosin and a "globular" Ca2+-sensitive protein complex called troponin. The structure of the tropomyosin filaments has now been determined by x-ray crystallography to approximately 15 A resolution. The complete sequence of alpha-tropomyosin is known; by using mercury markers on the cysteine residues the ends of the molecules in the filaments have been identified. Details of the coiled-coil structure have also been visualized by refinement of models against the diffraction data. The average pitch of the coiled coil is approximately 137 A, so that each tropomyosin molecule can make similar contacts with seven actin monomers. The electron density map also indicates that departures from the alpha-helical coiled coil occur in a few localized regions of the molecule, especially at the overlapping ends. Motions of tropomyosin in the crystal lattice are displaced by the character of the Bragg reflections and the strong diffuse scatter. These effects depend markedly on temperature. It appears that the molecular filaments fluctuate freely in a direction perpendicular to their axes. Moreover, the C-terminal half of the molecule "unfolds" to some degree at less than physiological temperatures. Crystallographic results on co-crystals of tropomyosin and a component of troponin (TnT) suggest that this subunit consists of structurally distinct domains, so that the troponin complex is not in fact simply "globular". The interactions of the extended alpha-helical region of TnT may "stiffen" tropomyosin and influence its motions. We picture the tropomyosin/troponin switch in muscle as a restless cable, perpetually making and breaking bonds as it vibrates on the thin filament. These movements of tropomyosin probably depend on two aspects of its design: the regular pattern of coiled-coil linkages with actin; and the aperiodic features that allow flexibility and motion.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson L., Nyström L. E., Lindberg U., Kannan K. K., Cid-Dresdner H., Lövgren S. Crystallization of a non-muscle actin. J Mol Biol. 1976 Aug 15;105(3):353–366. doi: 10.1016/0022-2836(76)90098-x. [DOI] [PubMed] [Google Scholar]
- Caspar D. L., Cohen C., Longley W. Tropomyosin: crystal structure, polymorphism and molecular interactions. J Mol Biol. 1969 Apr 14;41(1):87–107. doi: 10.1016/0022-2836(69)90128-4. [DOI] [PubMed] [Google Scholar]
- Cohen C., Caspar D. L., Parry D. A., Lucas R. M. Tropomyosin crystal dynamics. Cold Spring Harb Symp Quant Biol. 1972;36:205–216. doi: 10.1101/sqb.1972.036.01.028. [DOI] [PubMed] [Google Scholar]
- Cohen C., Longley W. Tropomyosin paracrystals formed by divalent cations. Science. 1966 May 6;152(3723):794–796. doi: 10.1126/science.152.3723.794. [DOI] [PubMed] [Google Scholar]
- Cohen C. The protein switch of muscle contraction. Sci Am. 1975 Nov;233(5):36–45. doi: 10.1038/scientificamerican1175-36. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Subunit structure and biological activity of tropomyosin B from different muscle types. Biochem J. 1972 Jul;128(3):106P–107P. doi: 10.1042/bj1280106pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBASHI S., EBASHI F. A NEW PROTEIN COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF MYOSIN B. J Biochem. 1964 Jun;55:604–613. doi: 10.1093/oxfordjournals.jbchem.a127933. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Pyun H. Y. Calcium binding by the troponin complex, and the purification and properties of troponin A. Biochim Biophys Acta. 1971 Mar 23;229(3):698–711. doi: 10.1016/0005-2795(71)90286-8. [DOI] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of muscle contraction: bindings of troponin and its components to actin and tropomyosin. Eur J Biochem. 1975 Mar 17;52(2):255–263. doi: 10.1111/j.1432-1033.1975.tb03993.x. [DOI] [PubMed] [Google Scholar]
- Huber R. Conformational flexibility in protein molecules. Nature. 1979 Aug 16;280(5723):538–539. doi: 10.1038/280538a0. [DOI] [PubMed] [Google Scholar]
- Johnson F., Smillie L. B. Rabbit skeletal alpha-tropomyosin chains are in register. Biochem Biophys Res Commun. 1975 Jun 16;64(4):1316–1322. doi: 10.1016/0006-291x(75)90836-0. [DOI] [PubMed] [Google Scholar]
- Katayama E. Interaction of troponin-I with troponin-T and its fragment. J Biochem. 1979 May;85(5):1379–1381. [PubMed] [Google Scholar]
- Lehrer S. S. Effects of an interchain disulfide bond on tropomyosin structure: intrinsic fluorescence and circular dichroism studies. J Mol Biol. 1978 Jan 15;118(2):209–226. doi: 10.1016/0022-2836(78)90413-8. [DOI] [PubMed] [Google Scholar]
- Lehrer S. S. Intramolecular crosslinking of tropomyosin via disulfide bond formation: evidence for chain register. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3377–3381. doi: 10.1073/pnas.72.9.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margossian S. S., Cohen C. Letter: Troponin subunit interactions. J Mol Biol. 1973 Dec 15;81(3):409–413. doi: 10.1016/0022-2836(73)90150-2. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D., Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975 Oct 25;98(2):293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- Mercola D., Bullard B., Priest J. Crystallisation of tropinin-C. Nature. 1975 Apr 17;254(5501):634–635. doi: 10.1038/254634a0. [DOI] [PubMed] [Google Scholar]
- Otsuk I. Distribution of troponin components in the thin filament studied by immunoelectron microscopy. J Biochem. 1975 Mar;77(3):633–639. doi: 10.1093/oxfordjournals.jbchem.a130765. [DOI] [PubMed] [Google Scholar]
- Otsuki I. Localization of troponin in thin filament and tropomyosin paracrystal. J Biochem. 1974 Apr;75(4):753–765. doi: 10.1093/oxfordjournals.jbchem.a130448. [DOI] [PubMed] [Google Scholar]
- Parry D. A. Analysis of the primary sequence of alpha-tropomyosin from rabbit skeletal muscle. J Mol Biol. 1975 Nov 5;98(3):519–535. doi: 10.1016/s0022-2836(75)80084-2. [DOI] [PubMed] [Google Scholar]
- Parry D. A. Movement of tropomyosin during regulation of vertebrate skeletal muscle: a simple physical model. Biochem Biophys Res Commun. 1976 Jan 26;68(2):323–328. doi: 10.1016/0006-291x(76)91146-3. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Johnson P., Carpenter M. R., Smillie L. B. Primary structure of rabbit skeletal muscle troponin-T. Sequence determination of the NH2-terminal fragment CB3 and the complete sequence of troponin-T. J Biol Chem. 1977 Feb 10;252(3):983–989. [PubMed] [Google Scholar]
- Pearlstone J. R., Smillie L. B. The binding site of skeletal alpha-tropomyosin on troponin-T. Can J Biochem. 1977 Oct;55(10):1032–1038. doi: 10.1139/o77-154. [DOI] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Lattman E. E., Cummins P., Lee K. Y., Cohen C. Crystal structure and molecular interactions of tropomyosin. Nature. 1979 Mar 29;278(5703):413–417. doi: 10.1038/278413a0. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Seidel J. C., Leavis P., Lehrer S. S., Gergely J. Effect of Ca2+ binding on troponin C. Changes in spin label mobility, extrinsic fluorescence, and sulfhydryl reactivity. J Biol Chem. 1976 Dec 10;251(23):7551–7556. [PubMed] [Google Scholar]
- Richardson J. S. beta-Sheet topology and the relatedness of proteins. Nature. 1977 Aug 11;268(5620):495–500. doi: 10.1038/268495a0. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Deficiency in histone acetylation in nontransforming host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1092–1096. doi: 10.1073/pnas.73.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodek J., Hodges R. S., Smillie L. B. Amino acid sequence of rabbit skeletal muscle alpha-tropomyosin. The COOH-terminal half (residues 142 to 284). J Biol Chem. 1978 Feb 25;253(4):1129–1136. [PubMed] [Google Scholar]
- Squire J. M. Muscle filament structure and muscle contraction. Annu Rev Biophys Bioeng. 1975;4(00):137–163. doi: 10.1146/annurev.bb.04.060175.001033. [DOI] [PubMed] [Google Scholar]
- Stone D., Smillie L. B. The amino acid sequence of rabbit skeletal alpha-tropomyosin. The NH2-terminal half and complete sequence. J Biol Chem. 1978 Feb 25;253(4):1137–1148. [PubMed] [Google Scholar]
- Ueno H. Binding of troponin components to tropomyosin fragments. J Biochem. 1978 Oct;84(4):1009–1012. doi: 10.1093/oxfordjournals.jbchem.a132182. [DOI] [PubMed] [Google Scholar]
- Vibert P. J., Haselgrove J. C., Lowy J., Poulsen F. R. Structural changes in actin-containing filaments of muscle. J Mol Biol. 1972 Nov 28;71(3):757–767. doi: 10.1016/s0022-2836(72)80036-6. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., McLachlan A. D. Structural homology of myosin alkali light chains, troponin C and carp calcium binding protein. Nature. 1974 Dec 20;252(5485):646–649. doi: 10.1038/252646a0. [DOI] [PubMed] [Google Scholar]
- Wegner A. Equilibrium of the actin-tropomyosin interaction. J Mol Biol. 1979 Jul 15;131(4):839–853. doi: 10.1016/0022-2836(79)90204-3. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Grand R. J. The amino acid sequence of troponin I from rabbit skeletal muscle. Biochem J. 1975 Aug;149(2):493–496. [PMC free article] [PubMed] [Google Scholar]
- Woods E. F. Molecular weight and subunit structure of tropomyosin B. J Biol Chem. 1967 Jun 25;242(12):2859–2871. [PubMed] [Google Scholar]