Abstract
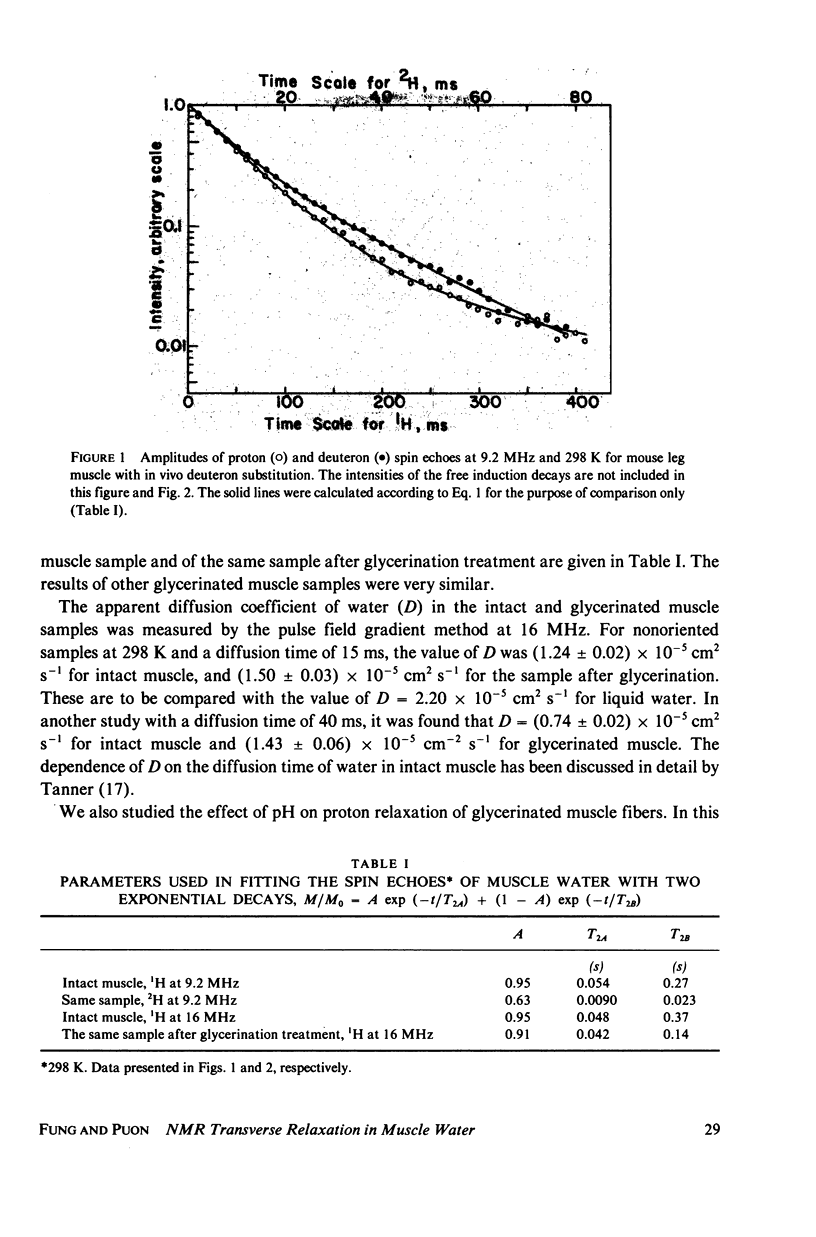
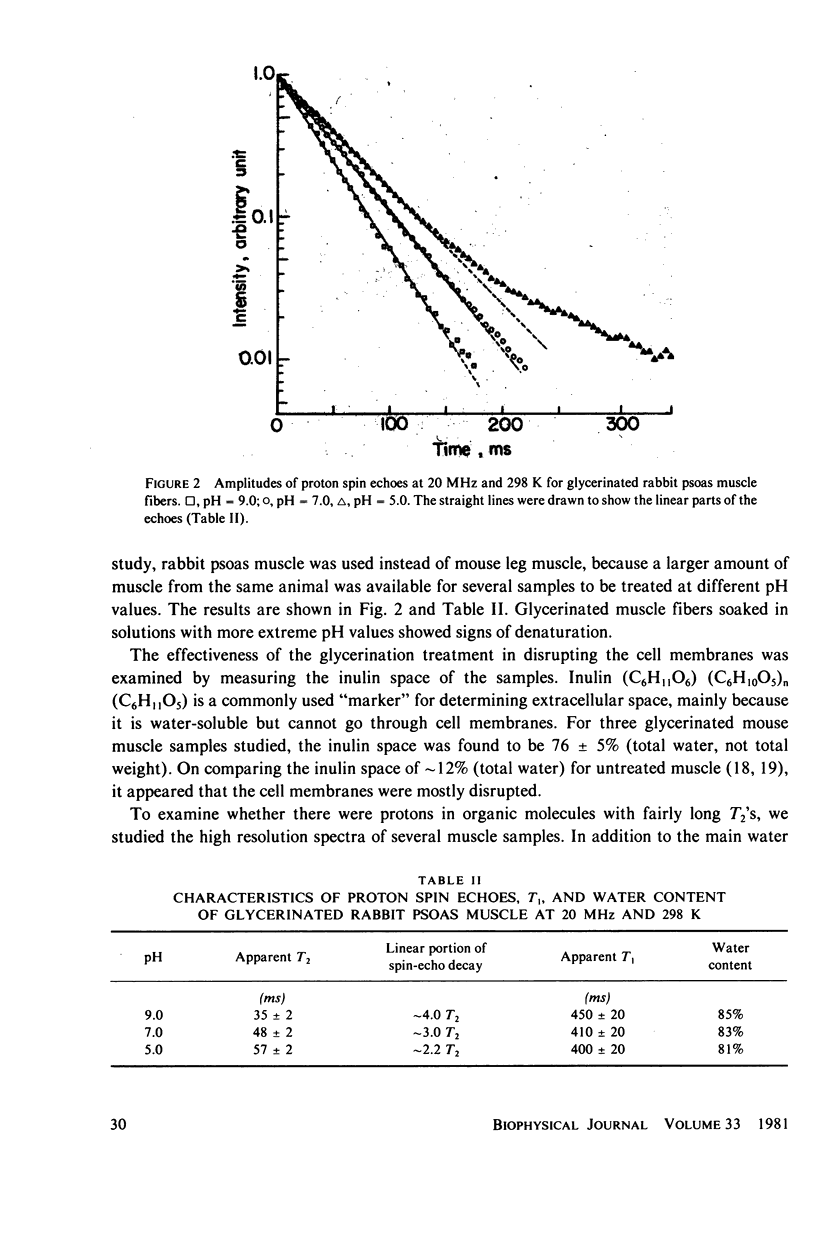
The origin of the nonexponentiality of proton spin echoes of skeletal muscle has been carefully examined. It is shown that the slowly decaying part of the proton spin echoes is not due to extracellular water. First, for muscle from mice with in vivo deuteration, the deuteron spin echoes were also nonexponential, but the slowly decaying part had a larger weighing factor. Second, for glycerinated muscle in which cell membranes were disrupted, the proton spin echoes were similar to those in intact muscle. Third, the nonexponentiality of the proton spin echoes in intact muscle increased when postmortem rigor set in. Finally, when the lifetimes of extracellular water and intracellular water were taken into account in the exchange, it was found that the two types of water would not give two resolvable exponentials with the observed decay constants. It is suggested that the unusually short T2's and the nonexponential character of the spin echoes of proton and deuteron in muscle water are mainly due to hydrogen exchange between water and functional groups in the protein filaments. These groups have large dipolar or quadrupolar splittings, and undergo hydrogen exchange with water at intermediate rates. The exchange processes and their effects on the spin echoes are pH-dependent. The dependence of transverse relaxation of pH was observed in glycerinated rabbit psoas muscle fibers.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belton P. S., Jackson R. R., Packer K. J. Pulsed NMR studies of water in striated muscle. I. Transverse nuclear spin relaxation times and freezing effects. Biochim Biophys Acta. 1972 Nov 24;286(1):16–25. doi: 10.1016/0304-4165(72)90084-0. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J., Kane F., O'reilly H. L. Volume of interfibre spaces in frog muscle and the calculation of concentrations in the fibre water. J Physiol. 1941 Jun 30;99(4):401–414. doi: 10.1113/jphysiol.1941.sp003911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Achlama A. M., Shporer M. The relationship between the transverse and longitudinal nuclear magnetic resonance relaxation rates of muscle water. Biophys J. 1978 Feb;21(2):127–136. doi: 10.1016/S0006-3495(78)85513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civan M. M., Shporer M. Pulsed nuclear magnetic resonance study of 17-O, 2-D, and 1-H of water in frog striated muscle. Biophys J. 1975 Apr;15(4):299–306. doi: 10.1016/S0006-3495(75)85820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegel J. G., Pintar M. M. Origin of the nonexponentiality of the water proton spin relaxations in tissues. Biophys J. 1975 Sep;15(9):855–860. doi: 10.1016/S0006-3495(75)85861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K. R., Resing H. A., Garroway A. N. Bounds on "bound water": transverse nuclear magnetic resonance relaxation in barnacle muscle. Science. 1976 Oct 15;194(4262):324–326. doi: 10.1126/science.968484. [DOI] [PubMed] [Google Scholar]
- Fuchs F. Cooperative interactions between calcium-binding sites on glycerinated muscle fibers. The influence of cross-bridge attachment. Biochim Biophys Acta. 1977 Nov 17;462(2):314–322. doi: 10.1016/0005-2728(77)90130-x. [DOI] [PubMed] [Google Scholar]
- Fung B. M. Carbon-13 and proton magnetic resonance of mouse muscle. Biophys J. 1977 Sep;19(3):315–319. doi: 10.1016/S0006-3495(77)85591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. M. Correlation of relaxation time with water content in muscle and brain tissues. Biochim Biophys Acta. 1977 Mar 29;497(1):317–322. doi: 10.1016/0304-4165(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Fung B. M., Durham D. L., Wassil D. A. The state of water in biological systems as studied by proton and deuterium relaxation. Biochim Biophys Acta. 1975 Jul 14;399(1):191–202. doi: 10.1016/0304-4165(75)90225-1. [DOI] [PubMed] [Google Scholar]
- Fung B. M., McGaughy T. W. Study of spin-lattice and spin-spin relaxation times of 1H, 2H, and 17O in muscular water. Biophys J. 1979 Nov;28(2):293–303. doi: 10.1016/S0006-3495(79)85177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B. M., McGaughy T. W. The state of water in muscle as studied by pulsed NMR. Biochim Biophys Acta. 1974 May 24;343(3):663–673. doi: 10.1016/0304-4165(74)90287-6. [DOI] [PubMed] [Google Scholar]
- Fung B. M. Orientation of water in striated frog muscle. Science. 1975 Nov 21;190(4216):800–802. doi: 10.1126/science.1198098. [DOI] [PubMed] [Google Scholar]
- Fung B. M. Proton and deuteron relaxation of muscle water over wide ranges of resonance frequencies. Biophys J. 1977 May;18(2):235–239. doi: 10.1016/S0006-3495(77)85610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. The double array of filaments in cross-striated muscle. J Biophys Biochem Cytol. 1957 Sep 25;3(5):631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlewood C. F., Chang D. C., Nichols B. L., Woessner D. E. Nuclear magnetic resonance transverse relaxation times of water protons in skeletal muscle. Biophys J. 1974 Aug;14(8):583–606. doi: 10.1016/S0006-3495(74)85937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasturi S. R., Chang D. C., Hazlewood C. F. Study of anisotropy in nuclear magnetic resonance relaxation times of water protons in skeletal muscle. Biophys J. 1980 Jun;30(3):369–381. doi: 10.1016/S0006-3495(80)85102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. T., Duff I. D., Derbyshire W., Blanshard J. M. An NMR investigation of rigor in porcine muscle. Biochim Biophys Acta. 1974 Aug 7;362(1):188–200. doi: 10.1016/0304-4165(74)90040-3. [DOI] [PubMed] [Google Scholar]
- Peemoeller H., Pintar M. M., Kydon D. W. Nuclear magnetic resonance analysis of water in natural and deuterated mouse muscle above and below freezing. Biophys J. 1980 Mar;29(3):427–435. doi: 10.1016/S0006-3495(80)85144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peemoeller H., Pintar M. M. Nuclear magnetic resonance multiwindow analysis of proton local fields and magnetization distribution in natural and deuterated mouse muscle. Biophys J. 1979 Nov;28(2):339–355. doi: 10.1016/S0006-3495(79)85181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Cameron I. R. ECS, intracellular pH, and electrolytes of cardiac and skeletal muscle. Am J Physiol. 1975 Nov;229(5):1299–1304. doi: 10.1152/ajplegacy.1975.229.5.1299. [DOI] [PubMed] [Google Scholar]
- Tanner J. E. Self diffusion of water in frog muscle. Biophys J. 1979 Oct;28(1):107–116. doi: 10.1016/S0006-3495(79)85162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


