Abstract
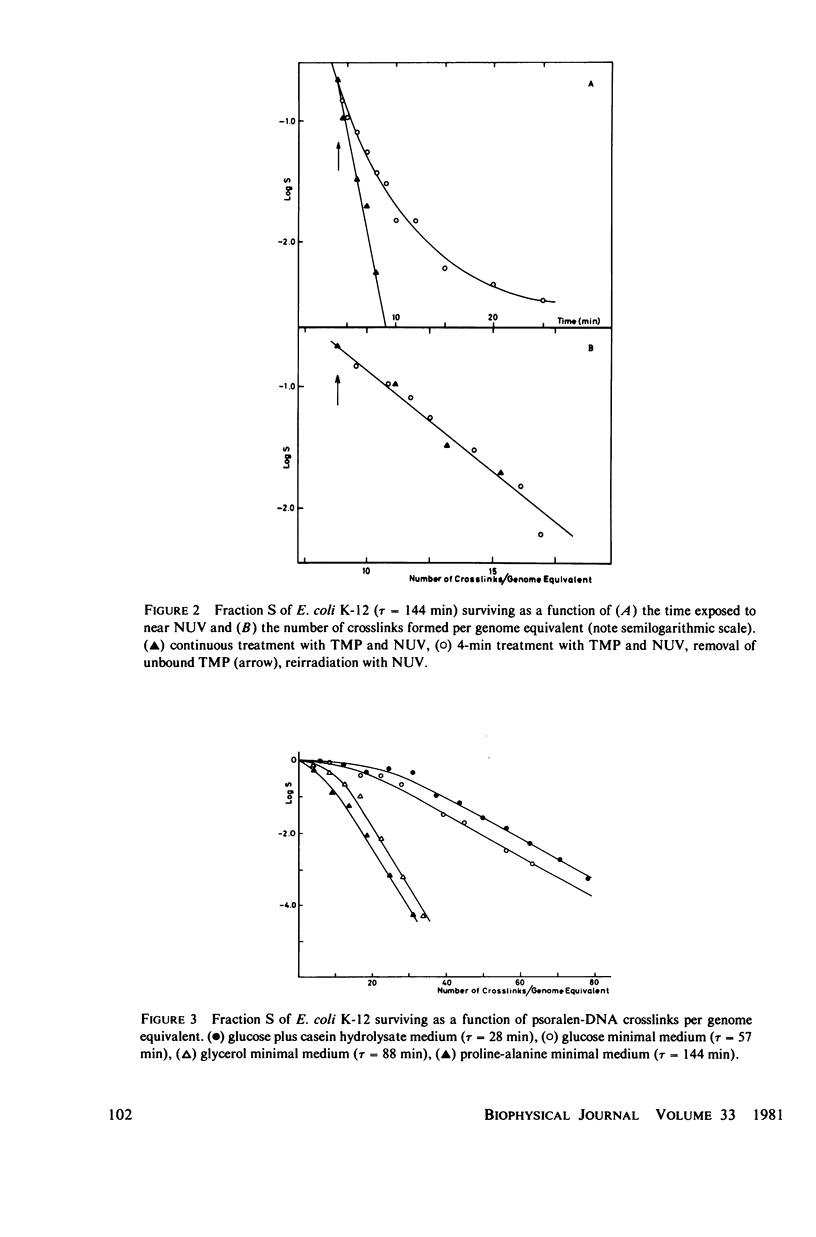
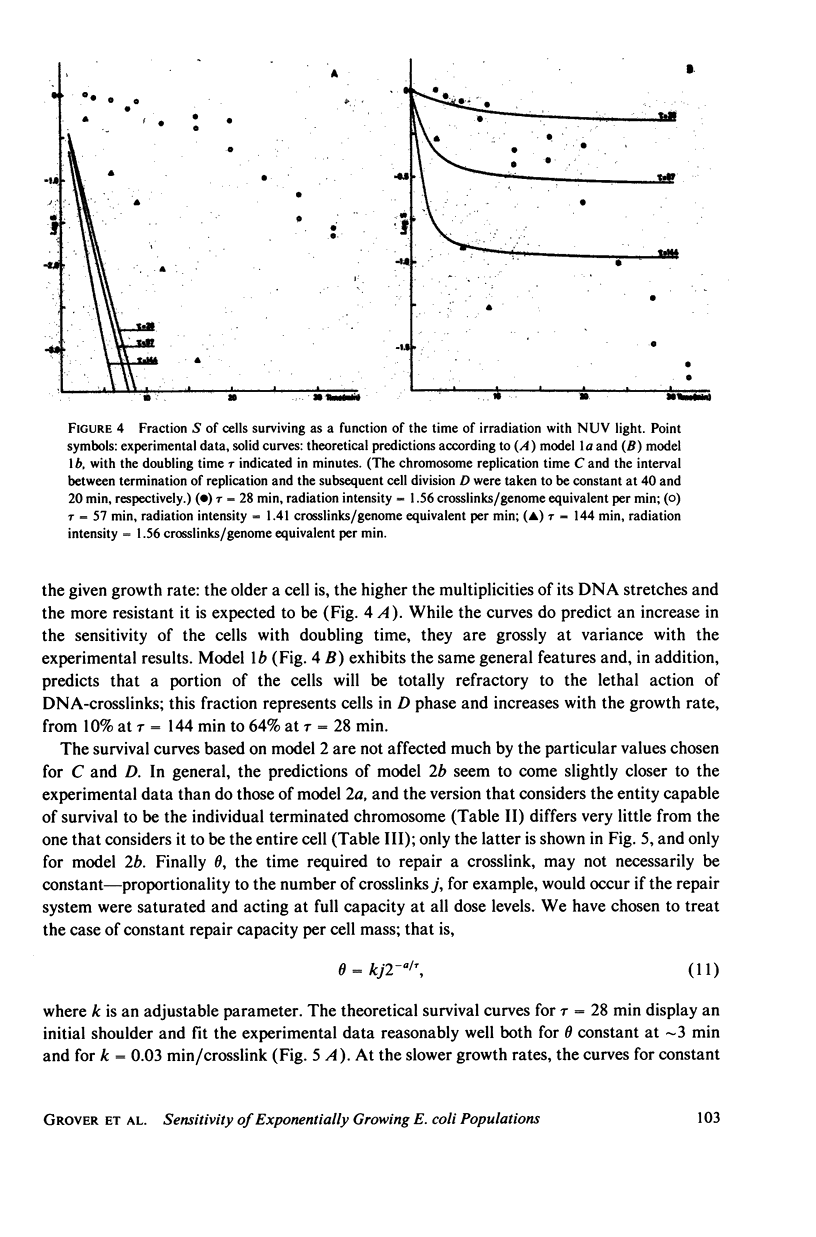
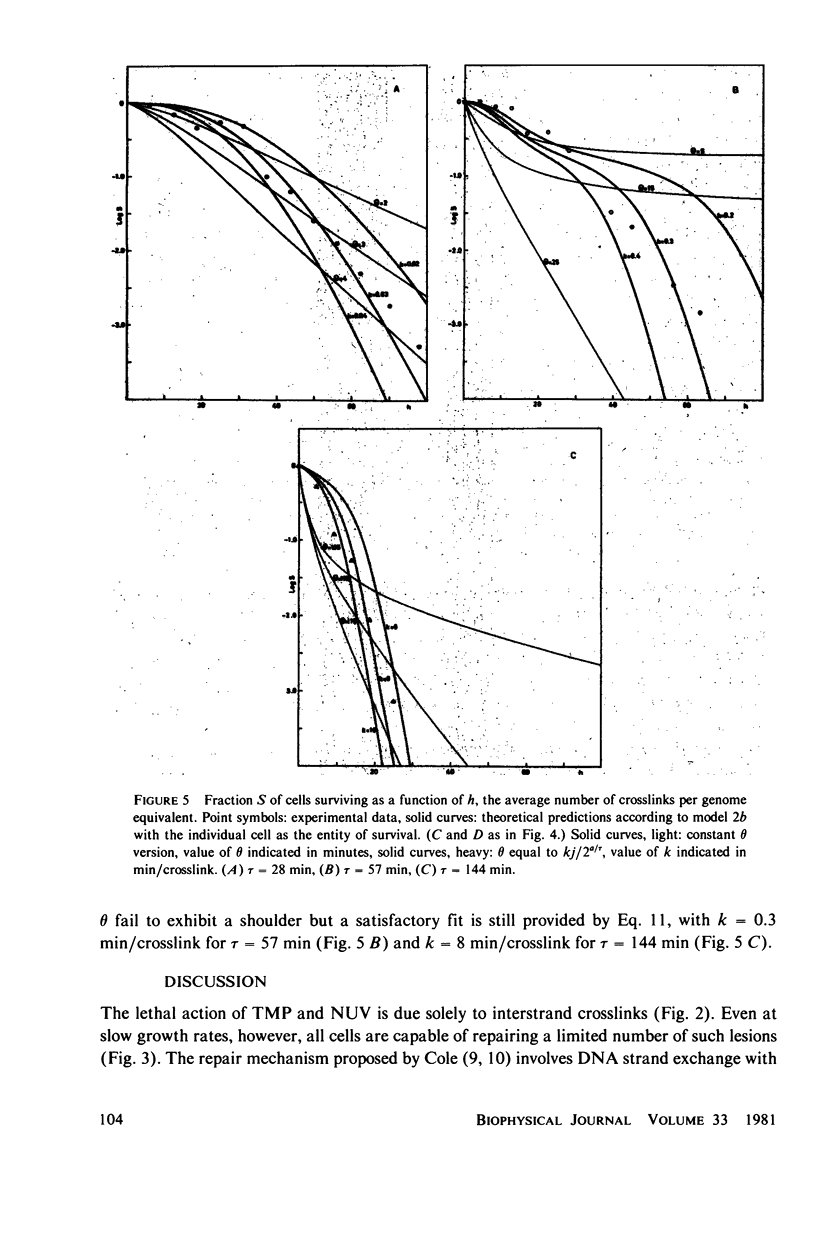
Experimental survival curves for Escherichia coli K 12 (CR 34) were determined after exposure to 4,5',8-trimethylpsoralen and near ultraviolet light. The lethal action was shown to arise exclusively from interstrand crosslinks, cell vulnerability increasing markedly with the doubling time of the culture. To account for these results, two quite different models are considered. The first assumes that a cell survives as long as at least one copy of its genome remains undamaged; a variant of this permits repair by DNA strand exchange. The second model allows for a limited period of time during which DNA repair can take place. A crosslink in a stretch of DNA due to be replicated within this interval constitutes a fatal lesion. Theoretical survival curves are computed for bacterial populations with defined age distributions and chromosome configurations. While the first model completely fails to provide a satisfactory description of the experimental results, the second model does predict the presence of a shoulder in the survival curves and, in one of its forms, it seems to agree rather well with the measured data over a wide range of crosslink concentrations and doubling times.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Hur E., Elkind M. M. Psoralen plus near ultraviolet light inactivation of cultured Chinese hamster cells and its relation to DNA cross-links. Mutat Res. 1973 Jun;18(3):315–324. doi: 10.1016/0027-5107(73)90216-9. [DOI] [PubMed] [Google Scholar]
- Ben-Hur E., Prager A., Riklis E. Measurement of DNA crosslinks by S1 nuclease: induction and repair in psoralen-plus-360 nm light treated Escherichia coli. Photochem Photobiol. 1979 May;29(5):921–924. doi: 10.1111/j.1751-1097.1979.tb07792.x. [DOI] [PubMed] [Google Scholar]
- Cassuto E., Gross N., Bardwell E., Howard-Flanders P. Genetic effects of photoadducts and photocross-links in the DNA of phage lambda exposed to 360 nm light and tri-methylpsoralen or khellin. Biochim Biophys Acta. 1977 Apr 19;475(4):589–600. doi: 10.1016/0005-2787(77)90319-7. [DOI] [PubMed] [Google Scholar]
- Chandler M., Bird R. E., Caro L. The replication time of the Escherichia coli K12 chromosome as a function of cell doubling time. J Mol Biol. 1975 May 5;94(1):127–132. doi: 10.1016/0022-2836(75)90410-6. [DOI] [PubMed] [Google Scholar]
- Chatterjee P. K., Cantor C. R. Photochemical production of psoralen - DNA monoadducts capable of subsequent photocrosslinking. Nucleic Acids Res. 1978 Oct;5(10):3619–3633. doi: 10.1093/nar/5.10.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J Bacteriol. 1971 Sep;107(3):846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Fujita H., Suzuki K. Effect of near-UV light on Escherichia coli in the presence of 8-methoxypsoralen: wavelength dependency of killing, induction of prophage, and mutation. J Bacteriol. 1978 Aug;135(2):354–362. doi: 10.1128/jb.135.2.354-362.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasin F., Hutchinson F. Repair of DNA double-strand breaks in Escherichia coli, which requires recA function and the presence of a duplicate genome. J Mol Biol. 1977 Oct 15;116(1):81–98. doi: 10.1016/0022-2836(77)90120-6. [DOI] [PubMed] [Google Scholar]
- Lane H. E., Denhardt D. T. The rep mutation. III. Altered structure of the replicating Escherichia coli chromosome. J Bacteriol. 1974 Nov;120(2):805–814. doi: 10.1128/jb.120.2.805-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. F., Bardwell E., Howard-Flanders P. Initiation of genetic exchanges in lambda phage--prophage crosses. Proc Natl Acad Sci U S A. 1977 Jan;74(1):291–295. doi: 10.1073/pnas.74.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL E. O. Growth rate and generation time of bacteria, with special reference to continuous culture. J Gen Microbiol. 1956 Dec;15(3):492–511. doi: 10.1099/00221287-15-3-492. [DOI] [PubMed] [Google Scholar]
- Rosenberger R. F., Grover N. B., Zaritsky A., Woldringh C. L. Surface growth in rod-shaped bacteria. J Theor Biol. 1978 Aug 21;73(4):711–721. doi: 10.1016/0022-5193(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Scott B. R., Pathak M. A., Mohn G. R. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39(1):29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Slater M., Schaechter M. Control of cell division in bacteria. Bacteriol Rev. 1974 Jun;38(2):199–221. doi: 10.1128/br.38.2.199-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Yoshikawa H. The chromosome of Bacillus subtilis. I. Theory of marker frequency analysis. Genetics. 1965 Oct;52(4):747–757. doi: 10.1093/genetics/52.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Replication time of the chromosome in thymineless mutants of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):65–74. doi: 10.1016/0022-2836(71)90447-5. [DOI] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L. Chromosome replication rate and cell shape in Escherichia coli: lack of coupling. J Bacteriol. 1978 Aug;135(2):581–587. doi: 10.1128/jb.135.2.581-587.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


