Abstract
In a previous paper, bioenergetic aspects of head-to-tail polymerization for a two-state actin ATPase cycle were discussed. In section 2, here, the steady-state polymer length distribution for this case is derived. The distribution has the same mathematical form as at equilibrium, but the parameters are different. In section 3, both bioenergetic topics and the polymer length distribution are considered for the more general and realistic case of a three-state actin ATPase cycle. Again, the mathematical form of the steady-state distribution is the same as at equilibrium, but the parameters are more complicated. In section 4, the question is examined of how much the mean and variance of a polymer length distribution, obtained from a finite experimental sample of polymer (aggregate) molecules, would be expected to deviate from the true mean and variance (from an infinite sample). Also considered briefly in section 4 is the effect of hard polymer-polymer interactions on the equilibrium polymer length distribution, at finite polymer concentrations.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergen L. G., Borisy G. G. Head-to-tail polymerization of microtubules in vitro. Electron microscope analysis of seeded assembly. J Cell Biol. 1980 Jan;84(1):141–150. doi: 10.1083/jcb.84.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
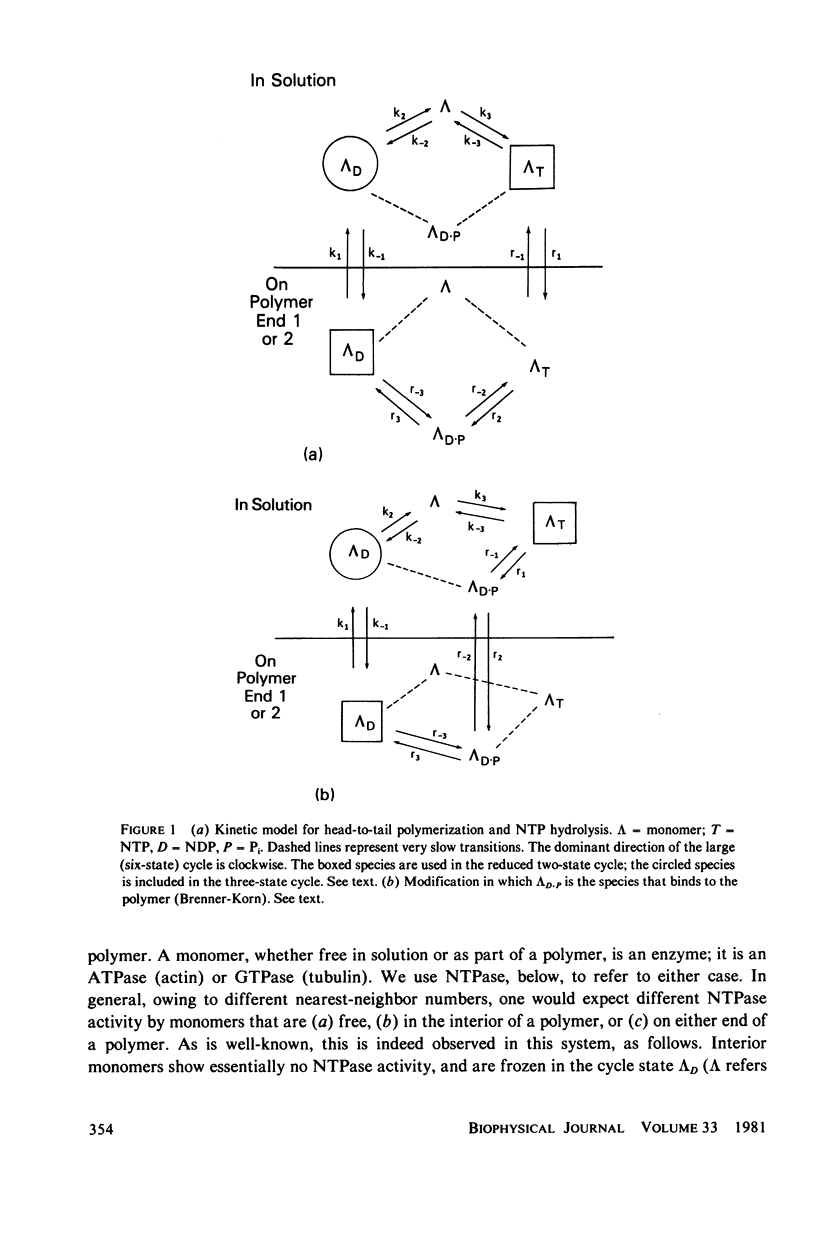
- Brenner S. L., Korn E. D. The effects of cytochalasins on actin polymerization and actin ATPase provide insights into the mechanism of polymerization. J Biol Chem. 1980 Feb 10;255(3):841–844. [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L., Chen Y. Cross-bridge model of muscle contraction. Quantitative analysis. Biophys J. 1980 Feb;29(2):195–227. doi: 10.1016/S0006-3495(80)85126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Cytochalasin B and the structure of actin gels. J Mol Biol. 1979 Nov 5;134(3):539–553. doi: 10.1016/0022-2836(79)90366-8. [DOI] [PubMed] [Google Scholar]
- Hill T. L. "Viral" expansion of enzyme flux and use of quasi-chemical approximation for two-state enzymes with enzyme-enzyme interactions. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5227–5230. doi: 10.1073/pnas.74.12.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
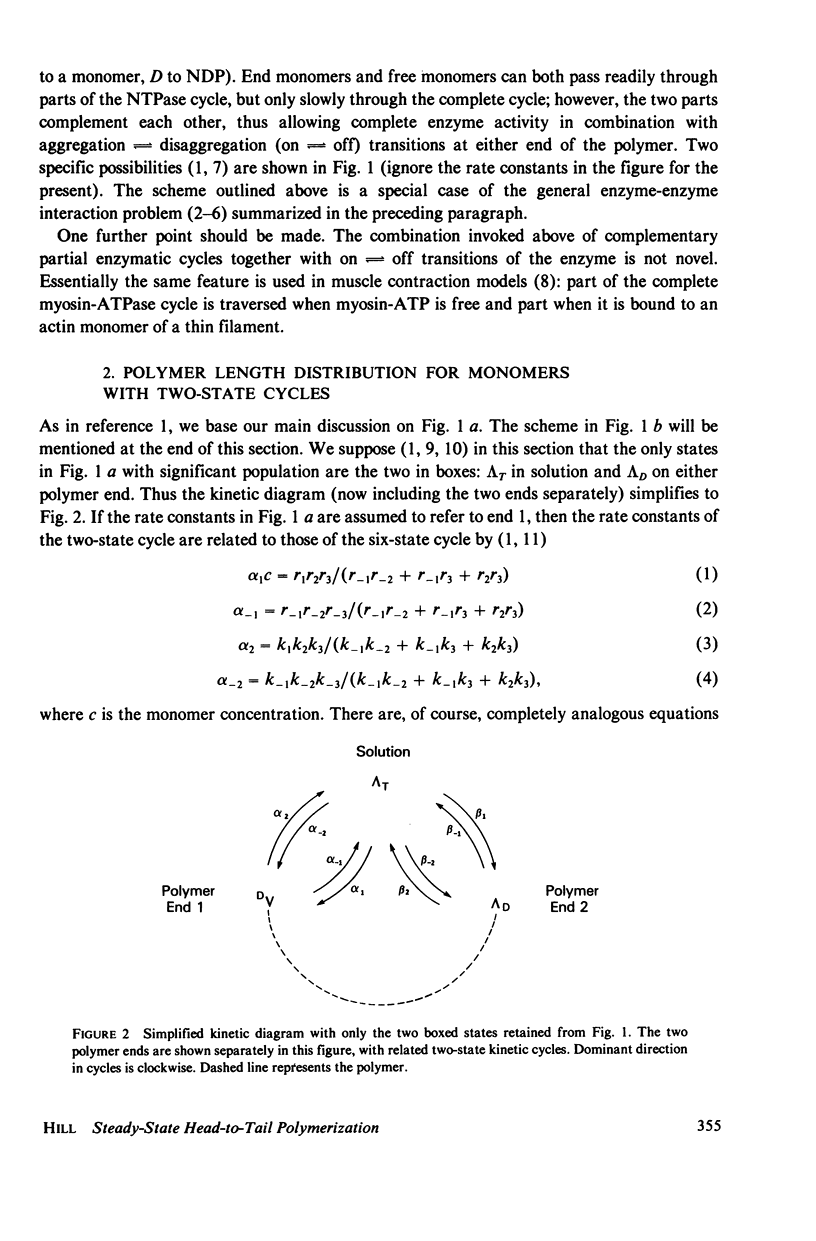
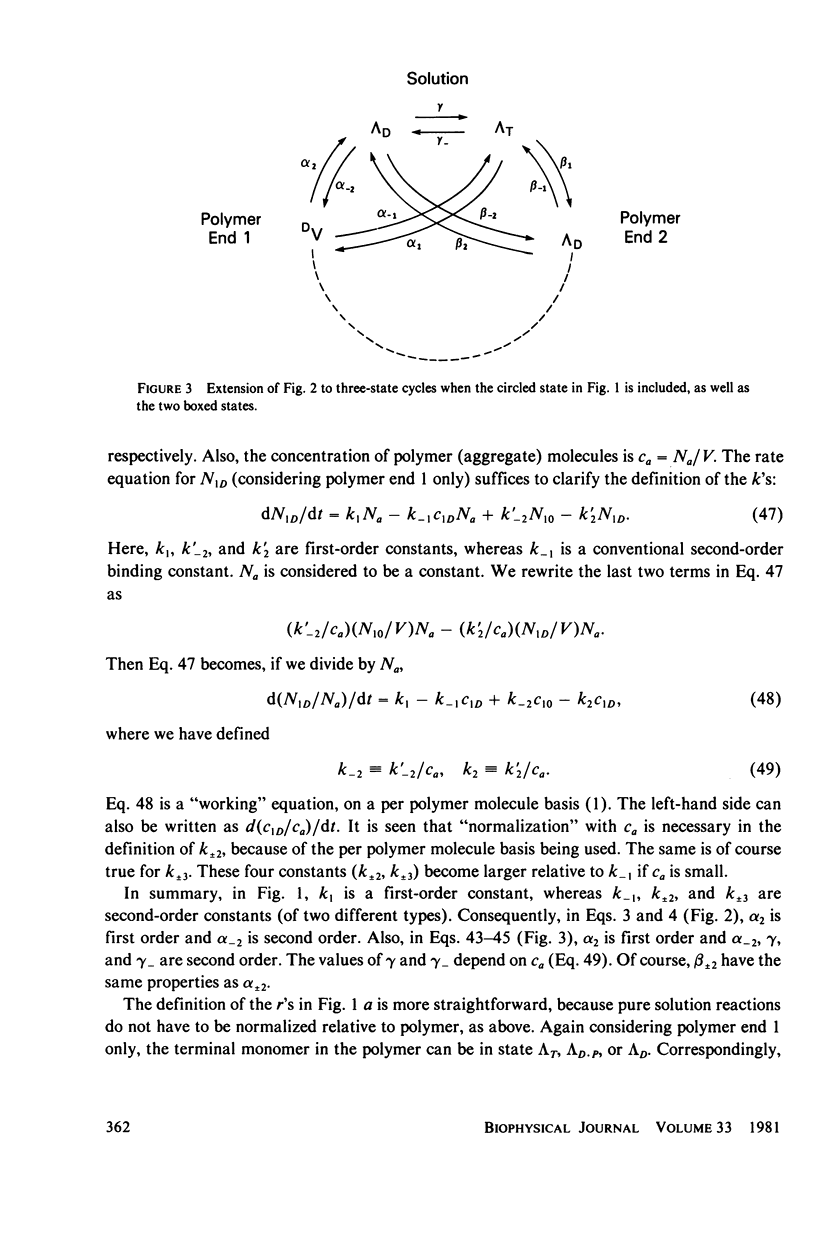
- Hill T. L. Bioenergetic aspects and polymer length distribution in steady-state head-to-tail polymerization of actin or microtubules. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4803–4807. doi: 10.1073/pnas.77.8.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. D. Interacting enzyme systems at steady state: location of the phase transition in approximations of the mean field type. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3015–3018. doi: 10.1073/pnas.75.7.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Further study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4111–4115. doi: 10.1073/pnas.74.10.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Levitzki A. Subunit neighbor interactions in enzyme kinetics: half-of-the-sites reactivity in a dimer. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5741–5745. doi: 10.1073/pnas.77.10.5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Theoretical study of the effect of enzyme-enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3632–3636. doi: 10.1073/pnas.74.9.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Unsymmetrical and concerted examples of the effect of enzyme--enzyme interactions on steady-state enzyme kinetics. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1101–1105. doi: 10.1073/pnas.75.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W. Implications of treadmilling for the stability and polarity of actin and tubulin polymers in vivo. J Cell Biol. 1980 Jul;86(1):330–334. doi: 10.1083/jcb.86.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidl C., Engel J. Exchange of ADP, ATP and 1: N6-ethenoadenosine 5'-triphosphate at G-actin. Equilibrium and kinetics. Eur J Biochem. 1979 Nov 1;101(1):163–169. doi: 10.1111/j.1432-1033.1979.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]


