Abstract
It is now more than 20 years since Davidson and collaborators (1957, Biochim. Biophys, Acta. 26:370-373; J. Mol. Biol. 1:190-191) applied the theoretical ideas of Bloembergen et al. (1948. Phys. Rev. 73:679-712) on outer sphere magnetic relaxation of solvent protons to studies of solutions of methemoglobin. From then on, there has been debate regarding the relative contributions to paramagnetic solvent proton relaxation by inner sphere (ligand-exchange) effects and by outer sphere (diffusional) effects in methemoglobin solutions. Gupta and Mildvan (1975. J. Biol. Chem 250:146-253) extended the early measurements, attributed the relatively small paramagnetic effects to exchange with solvent of the water ligand of the heme-Fe3+ ion, and interpreted their data to indicate cooperativity and an alkaline Bohr effect in the presence of inositol hexaphosphate. They neglected the earlier discussions entirely, and made no reference to outer sphere effects. We have measured the relaxation rate of solvent protons as a function of magnetic field for solutions of methemoglobin, under a variety of conditions of pH and temperature, and have given careful consideration to the relatively large diamagnetic corrections that are necessary by making analogous measurements on oxyhemoglobin, carbonmonoxyhemoglobin, and cyano- and azide-methemoglobin. (The latter two, because of their short electronic relaxation times, behave as though diamagnetic). We show that the paramagnetic contribution to solvent relaxation can be dominated by outer sphere effects, a result implying that many conclusions, including those of Gupta and Mildvan, require reexamination. Finally, we present data for fluoro-methemoglobin, which relaxes solvent protons an order of magnitude better than does methemoglobin. Here one has a startling breakdown of the dogma that has been the basis for interpreting many ligand-replacement studies; in contrast to the prevailing view that replacement of a water ligand of a protein-bound paramagnetic ion by another ligand should decrease relaxation rates, replacement of H2O by F- increases the relaxation rate drastically. The data can all be reconciled, however, with what is anticipated from knowledge of ligand interactions in the heme region.
Full text
PDF


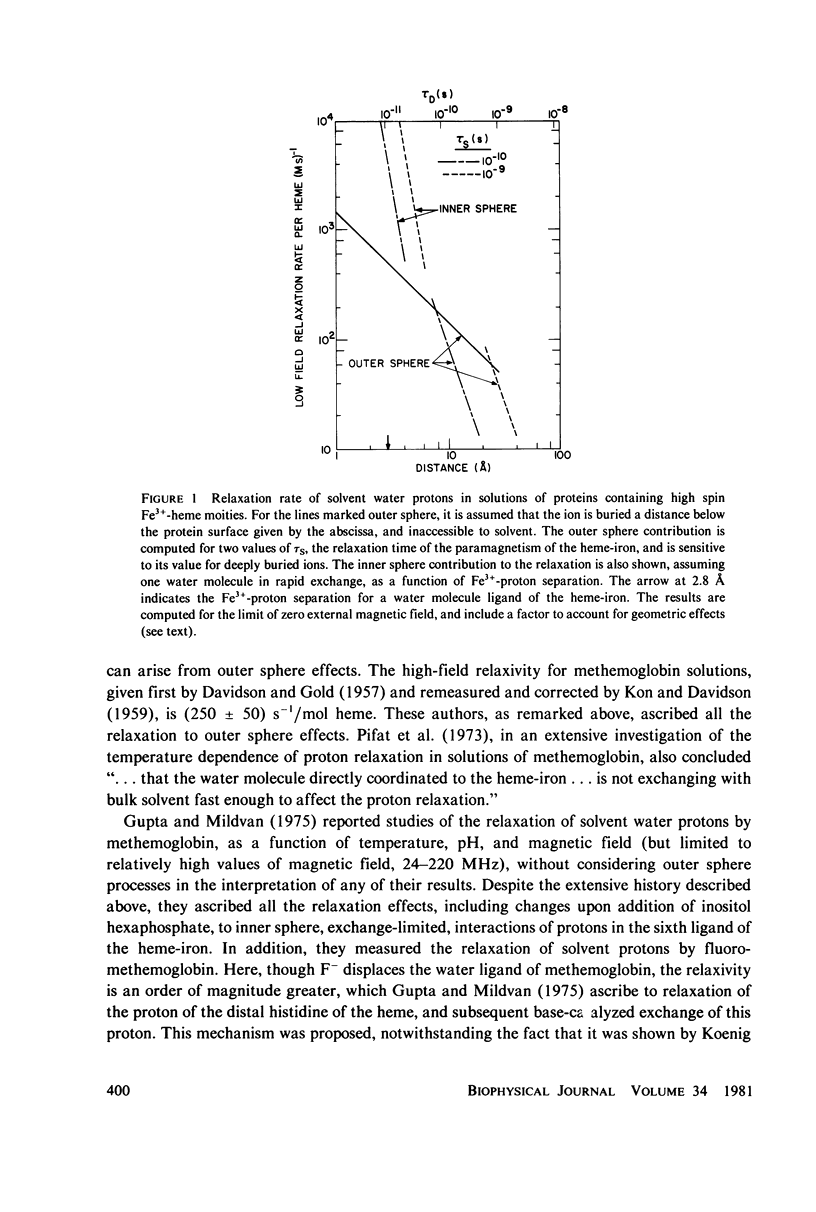


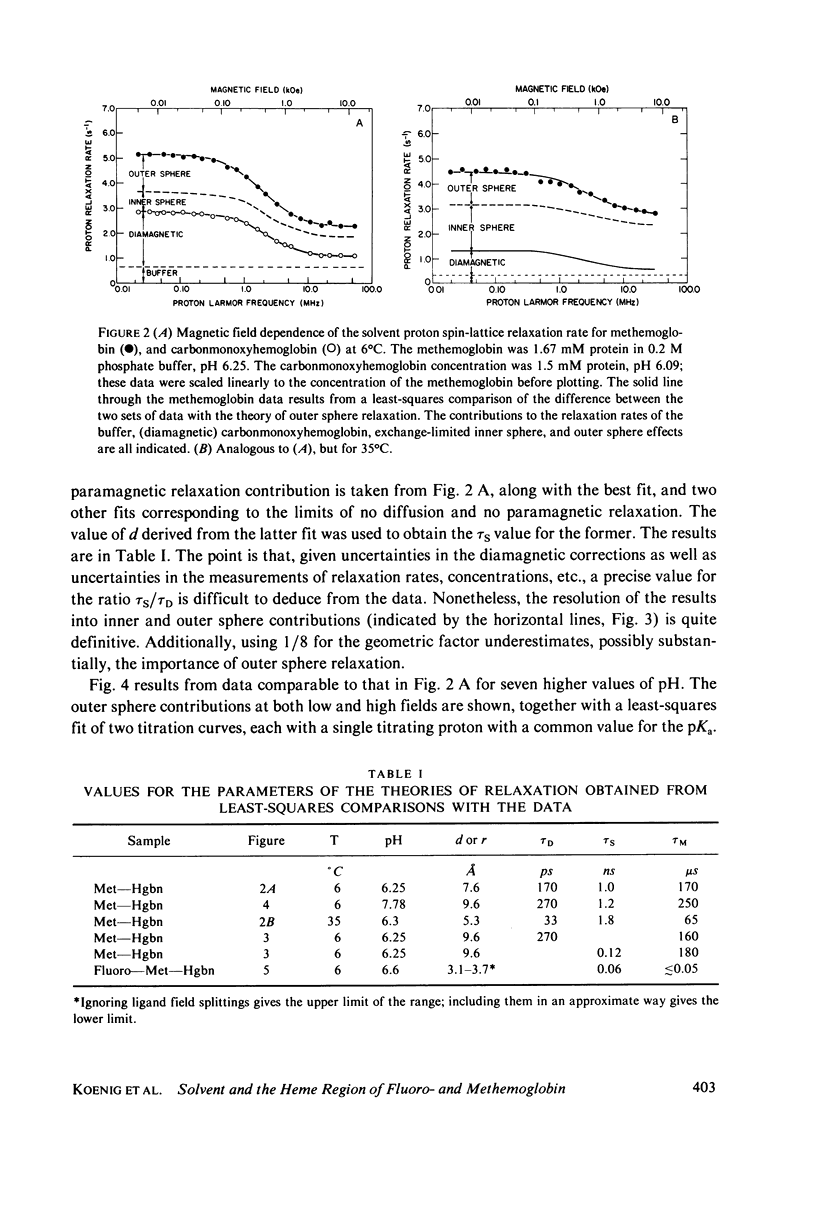
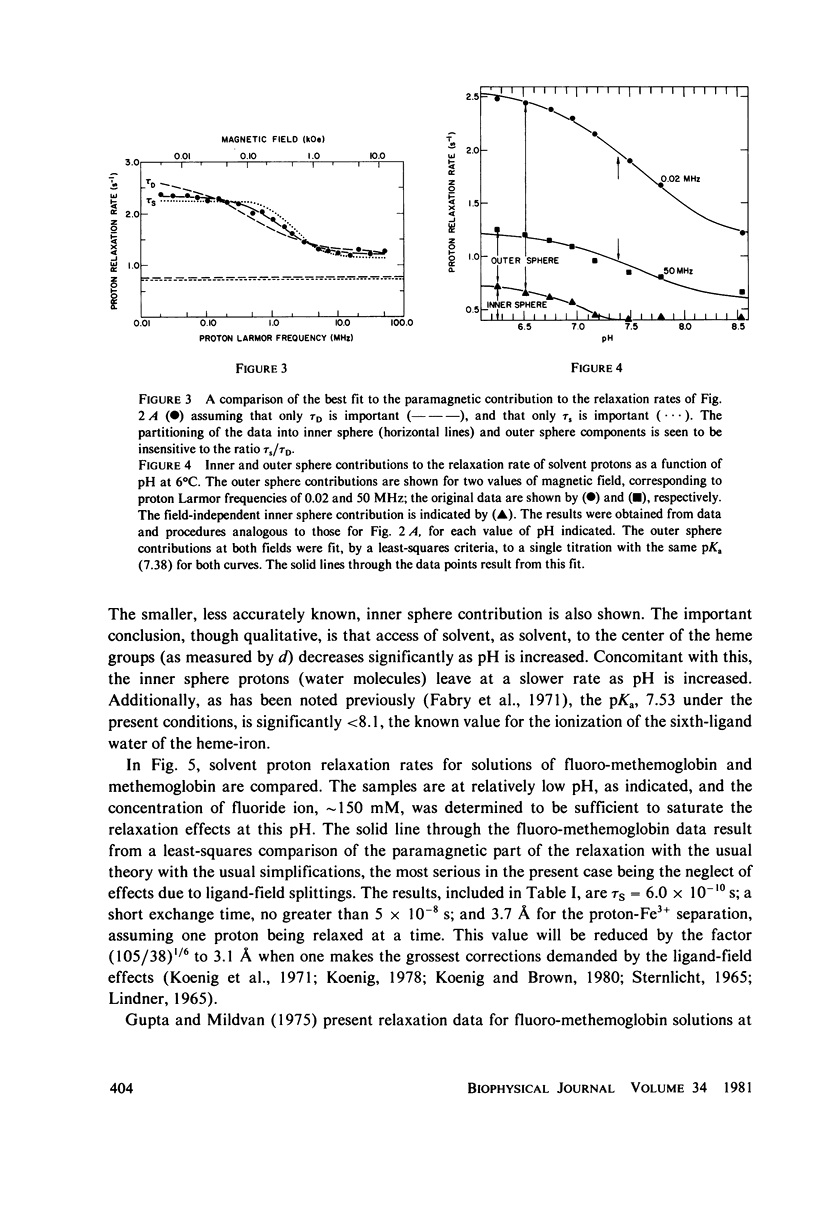
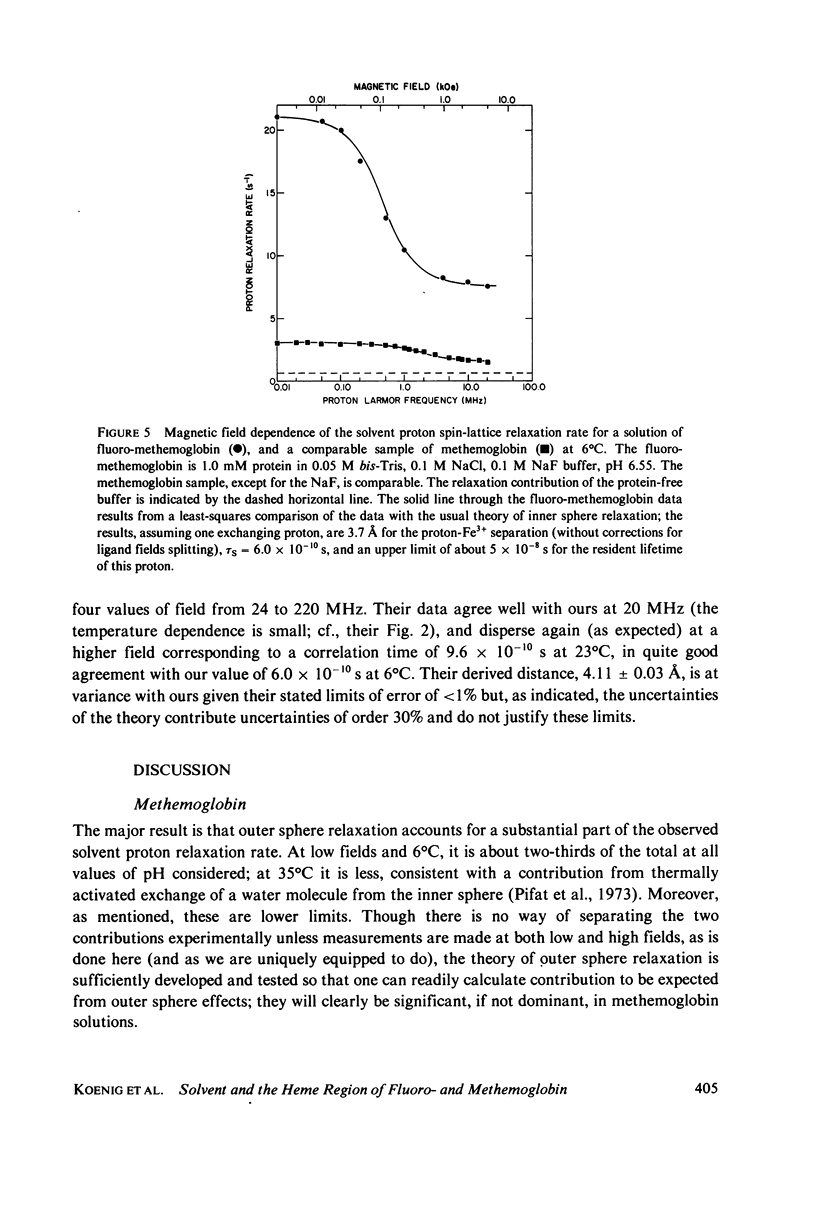



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher S. A., Schuster T. M. Resonance Raman examination of axial ligand bonding and spin-state equilibria in metmyoglobin hydroxide and other heme derivatives. Biochemistry. 1979 Nov 27;18(24):5377–5387. doi: 10.1021/bi00591a019. [DOI] [PubMed] [Google Scholar]
- Asher S. A., Vickery L. E., Schuster T. M., Sauer K. Resonance Raman spectra of methemoglobin derivatives. Selective enhancement of axial ligand vibrations and lack of an effect of inositol hexaphosphate. Biochemistry. 1977 Dec 27;16(26):5849–5856. doi: 10.1021/bi00645a032. [DOI] [PubMed] [Google Scholar]
- DAVIDSON N., GOLD R. The nuclear magnetic relaxation time of water protons in ferrihemoglobin solutions. Biochim Biophys Acta. 1957 Nov;26(2):370–373. doi: 10.1016/0006-3002(57)90018-5. [DOI] [PubMed] [Google Scholar]
- Deatherage J. F., Loe R. S., Moffat K. Structure of fluoride methemoglobin. J Mol Biol. 1976 Jul 5;104(3):723–728. doi: 10.1016/0022-2836(76)90131-5. [DOI] [PubMed] [Google Scholar]
- Fabry T. L., Reich H. A. The role of water in deoxygenated hemoglobin solutions. Biochem Biophys Res Commun. 1966 Mar 22;22(6):700–703. doi: 10.1016/0006-291x(66)90204-x. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Mildvan A. S. Nuclear relaxation studies on human methemoglobin. Observation of cooperativity and alkaline Bohr effect with inositol hexaphosphate. J Biol Chem. 1975 Jan 10;250(1):246–253. [PubMed] [Google Scholar]
- Hallenga K., Koenig S. H. Protein rotational relaxation as studied by solvent 1H and 2H magnetic relaxation. Biochemistry. 1976 Sep 21;15(19):4255–4264. doi: 10.1021/bi00664a019. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., 3rd H 2 CO 3 as substrate for carbonic anhydrase in the dehydration of HCO 3 . Proc Natl Acad Sci U S A. 1972 Sep;69(9):2422–2425. doi: 10.1073/pnas.69.9.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D. Anomalous relaxation of water protons in solutions of copper-containing proteins. Ann N Y Acad Sci. 1973 Dec 31;222:752–763. doi: 10.1111/j.1749-6632.1973.tb15302.x. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Brown R. D., Studebaker J. On the interpretation of solvent proton magnetic relaxation data with particular application to the structure of the active site of Mn-carboxypeptidase A. Cold Spring Harb Symp Quant Biol. 1972;36:551–559. doi: 10.1101/sqb.1972.036.01.069. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Bryant R. G., Hallenga K., Jacob G. S. Magnetic cross-relaxation among protons in protein solutions. Biochemistry. 1978 Oct 3;17(20):4348–4358. doi: 10.1021/bi00613a037. [DOI] [PubMed] [Google Scholar]
- Koenig S. H., Schillinger W. E. Nuclear magnetic relaxation dispersion in protein solutions. II. Transferrin. J Biol Chem. 1969 Dec 10;244(23):6520–6526. [PubMed] [Google Scholar]
- Lindstrom T. R., Koenig S. H., Boussios T., Bertles J. F. Intermolecular interactions of oxygenated sickle hemoglobin molecules in cells and cell-free solutions. Biophys J. 1976 Jun;16(6):679–689. doi: 10.1016/S0006-3495(76)85721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifat G., Maricić S., Grandja S. A proton magnetic relaxation study of human ferrihaemoglobin in aqueous salt solutions. Biopolymers. 1973 Apr;12(4):905–920. doi: 10.1002/bip.1973.360120418. [DOI] [PubMed] [Google Scholar]


