Abstract
The effect of Ca ion concentration on cross-bridge kinetics in a small bundle (one to three fibers) of chemically skinned rabbit psoas muscle is studied. The length of the muscle is oscillated in small amplitude sine waves (0.2% L0 peak-to-peak) at varying frequencies (0.125 -- 167 Hz), and the resulting amplitude and phase shift in tension are measured. The frequency response function (complex stiffness) thus obtained can be divided into three parts, which we name process (A) (centered at 1 Hz), process (B) (3--17 Hz), and process (C) (50 Hz). Process (B), which represents oscillatory work, further splits into two processes (B' and B) at partial Ca activation (less than 50% P0), where the phase-frequency plot appears W-shaped. The slower of the two processes (B') disappears by full activation, at which time the plot appears V-shaped. The characteristic frequencies associated with the minima of the plot do not shift in a graded way with Ca concentration, indicating that there is no change in apparent rate constants. Apparent rate constants of processes (A) and (C) are minimally affected by Ca. The above results are not altered when ionic strength is changed between 128 and 265 mM. We propose that activated thin filaments can have two "on" states and that Ca concentration controls the distribution of these two states. This mechanism generally supports the "switch" hypothesis of Ca regulation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott R. H. The effects of fibre length and calcium ion concentration on the dynamic response of glycerol extracted insect fibrillar muscle. J Physiol. 1973 Jun;231(2):195–208. doi: 10.1113/jphysiol.1973.sp010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April E., Brandt P. W., Reuben J. P., Grundfest H. Muscle contraction: the effect of ionic strength. Nature. 1968 Oct 12;220(5163):182–184. doi: 10.1038/220182a0. [DOI] [PubMed] [Google Scholar]
- Brandt P. W., Cox R. N., Kawai M. Can the binding of Ca2+ to two regulatory sites on troponin C determine the steep pCa/tension relationship of skeletal muscle? Proc Natl Acad Sci U S A. 1980 Aug;77(8):4717–4720. doi: 10.1073/pnas.77.8.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clerck N. M., Claes V. A., Brutsaert D. L. Force velocity relations of single cardiac muscle cells: calcium dependency. J Gen Physiol. 1977 Feb;69(2):221–241. doi: 10.1085/jgp.69.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F. Striated muscle. Annu Rev Physiol. 1974;36:461–502. doi: 10.1146/annurev.ph.36.030174.002333. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Godt R. E., Donaldson S. K., Harris C. E. Tension in skinned frog muscle fibers in solutions of varying ionic strength and neutral salt composition. J Gen Physiol. 1973 Nov;62(5):550–574. doi: 10.1085/jgp.62.5.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati J., Podolsky R. J. Contraction transients of skinned muscle fibers: effects of calcium and ionic strength. J Gen Physiol. 1978 Nov;72(5):701–715. doi: 10.1085/jgp.72.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F. Muscular contraction. J Physiol. 1974 Nov;243(1):1–43. [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophys J. 1969 Apr;9(4):547–570. doi: 10.1016/S0006-3495(69)86403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. Effects of calcium and ionic strength on shortening velocity and tension development in frog skinned muscle fibres. J Physiol. 1981 Feb;311:179–199. doi: 10.1113/jphysiol.1981.sp013580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J., Moss R. L. The concept of active state in striated muscle. Circ Res. 1976 Feb;38(2):53–59. doi: 10.1161/01.res.38.2.53. [DOI] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
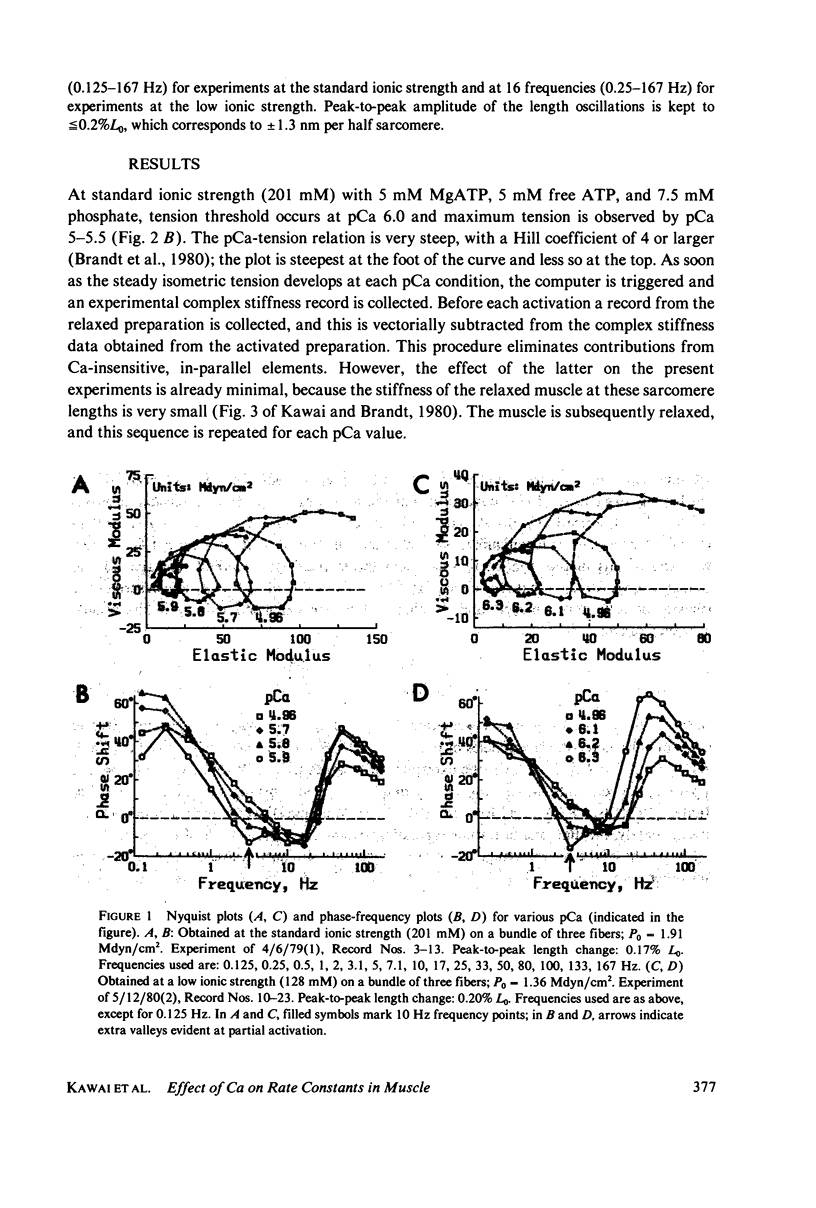
- Kawai M., Brandt P. W. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980 Sep;1(3):279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kawai M., Brandt P., Orentlicher M. Dependence of energy transduction in intact skeletal muscles on the time in tension. Biophys J. 1977 May;18(2):161–172. doi: 10.1016/S0006-3495(77)85605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M. Head rotation or dissociation? A study of exponential rate processes in chemically skinned rabbit muscle fibers when MgATP concentration is changed. Biophys J. 1978 Apr;22(1):97–103. doi: 10.1016/S0006-3495(78)85473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Podolsky R. J., Teichholz L. E. The relation between calcium and contraction kinetics in skinned muscle fibres. J Physiol. 1970 Nov;211(1):19–35. doi: 10.1113/jphysiol.1970.sp009263. [DOI] [PMC free article] [PubMed] [Google Scholar]
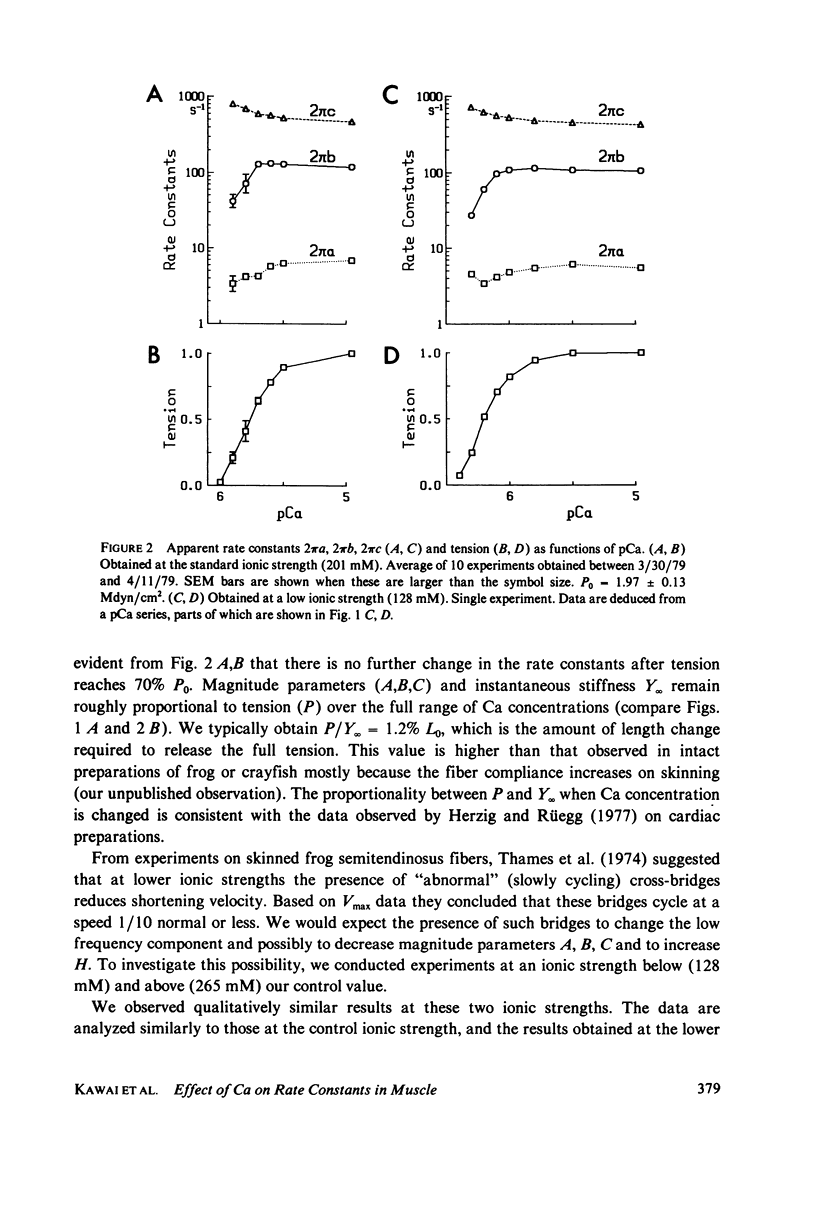
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Pringle J. W. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967;17:1–60. doi: 10.1016/0079-6107(67)90003-x. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G. Calcium regulation of muscle contraction. Biophys J. 1975 Jul;15(7):707–723. doi: 10.1016/S0006-3495(75)85849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thames M. D., Teichholz L. E., Podolsky R. J. Ionic strength and the contraction kinetics of skinned muscle fibers. J Gen Physiol. 1974 Apr;63(4):509–530. doi: 10.1085/jgp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson J., White D. C. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Weber A., Murray J. M. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973 Jul;53(3):612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- Wise R. M., Rondinone J. F., Briggs F. N. Effect of calcium on force-velocity characteristics of glycerinated skeletal muscle. Am J Physiol. 1971 Oct;221(4):973–979. doi: 10.1152/ajplegacy.1971.221.4.973. [DOI] [PubMed] [Google Scholar]


