Abstract
Least-squares analysis of experimental data from the analytical ultracentrifuge is discussed in detail, with particular attention to the use of interference optics in studying nonideal self-associating macromolecular systems. Several samples are given that describe the application of the technique, the expected precision of the results, and some of its limitations. A FORTRAN IV computer program is available from the authors.
Full text
PDF





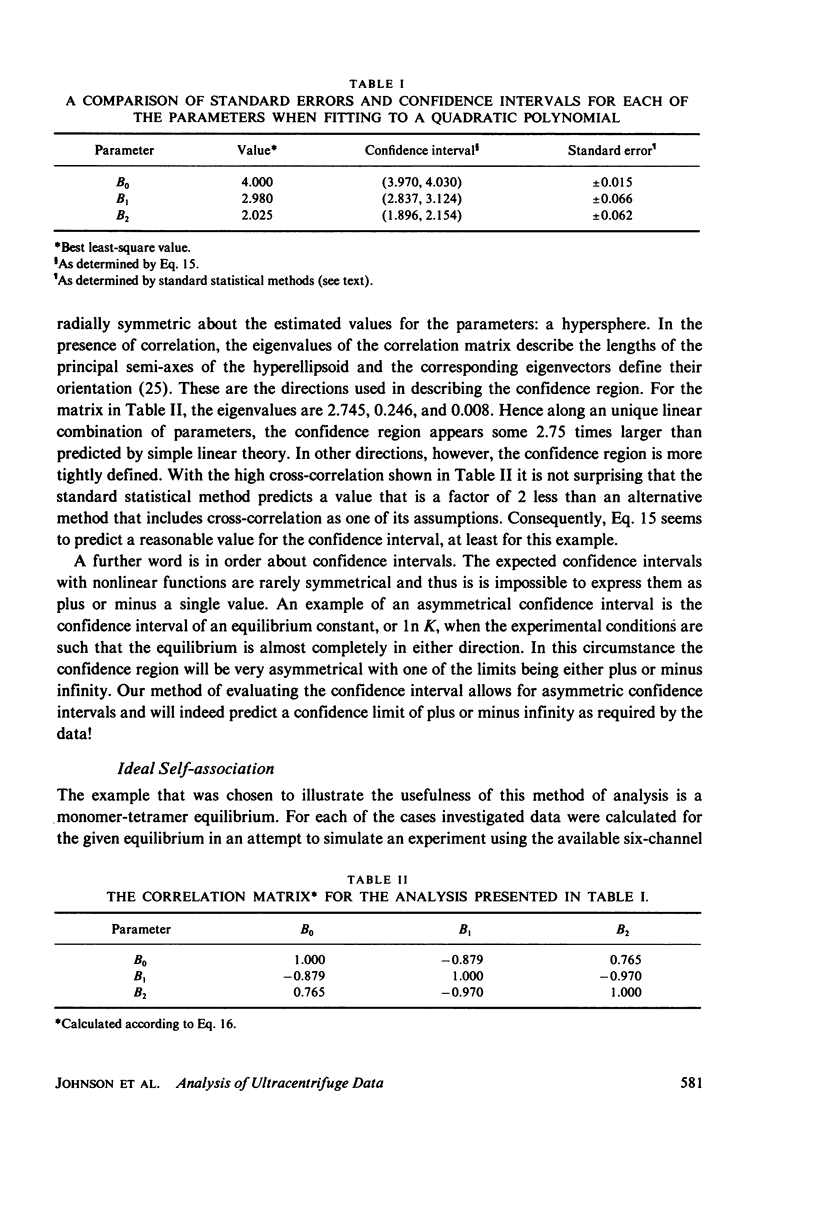
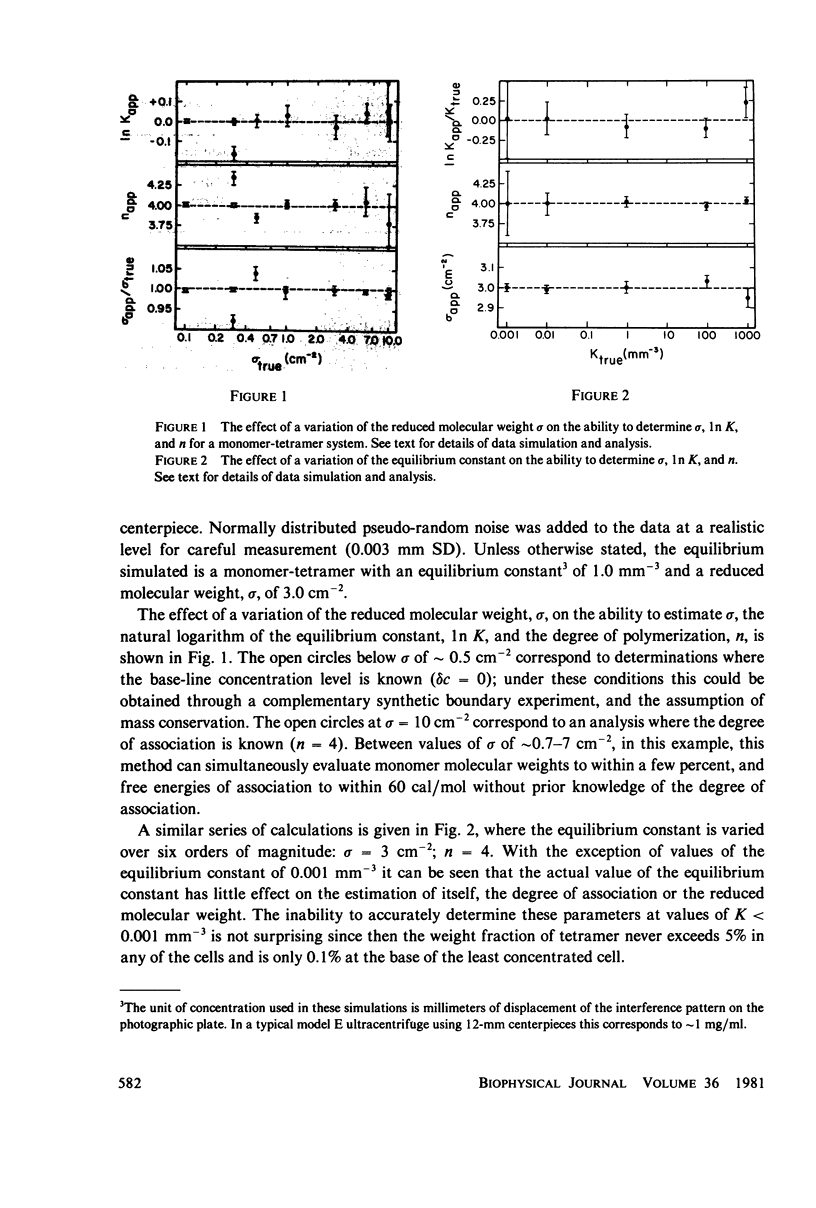
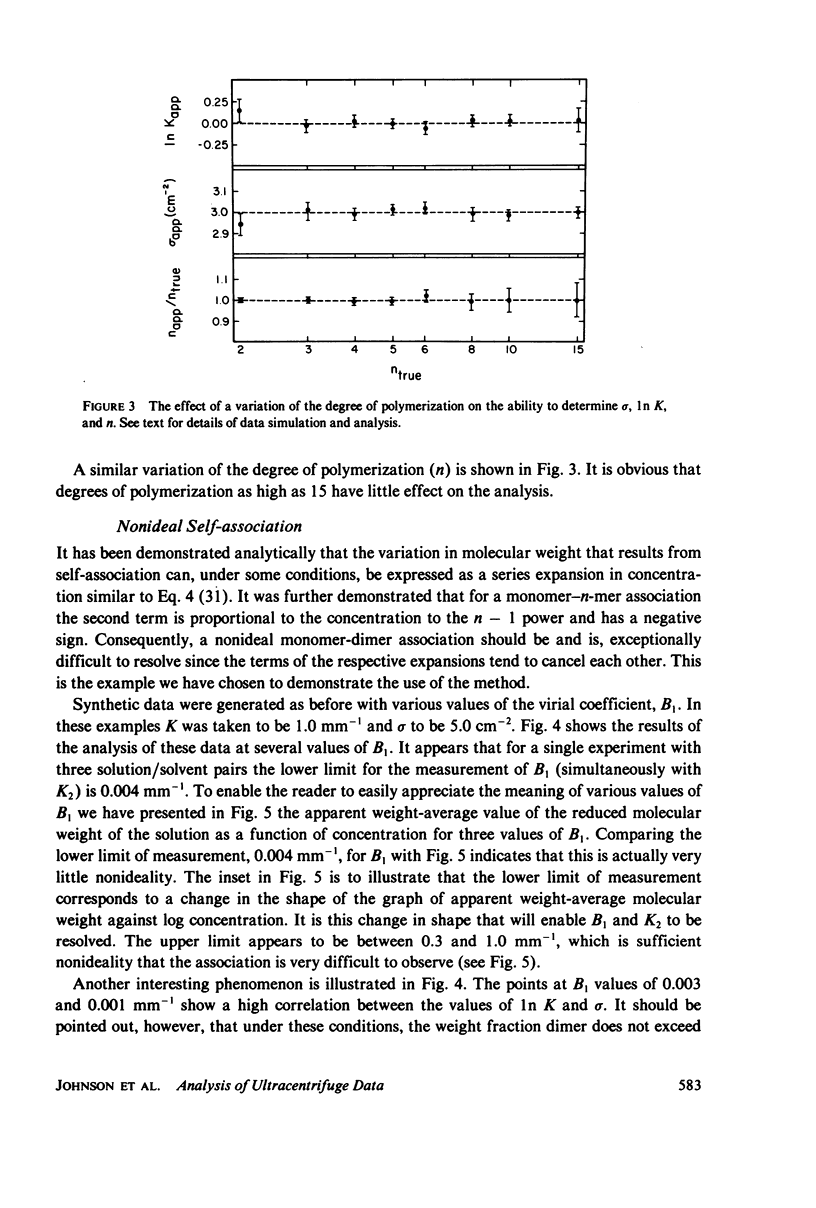
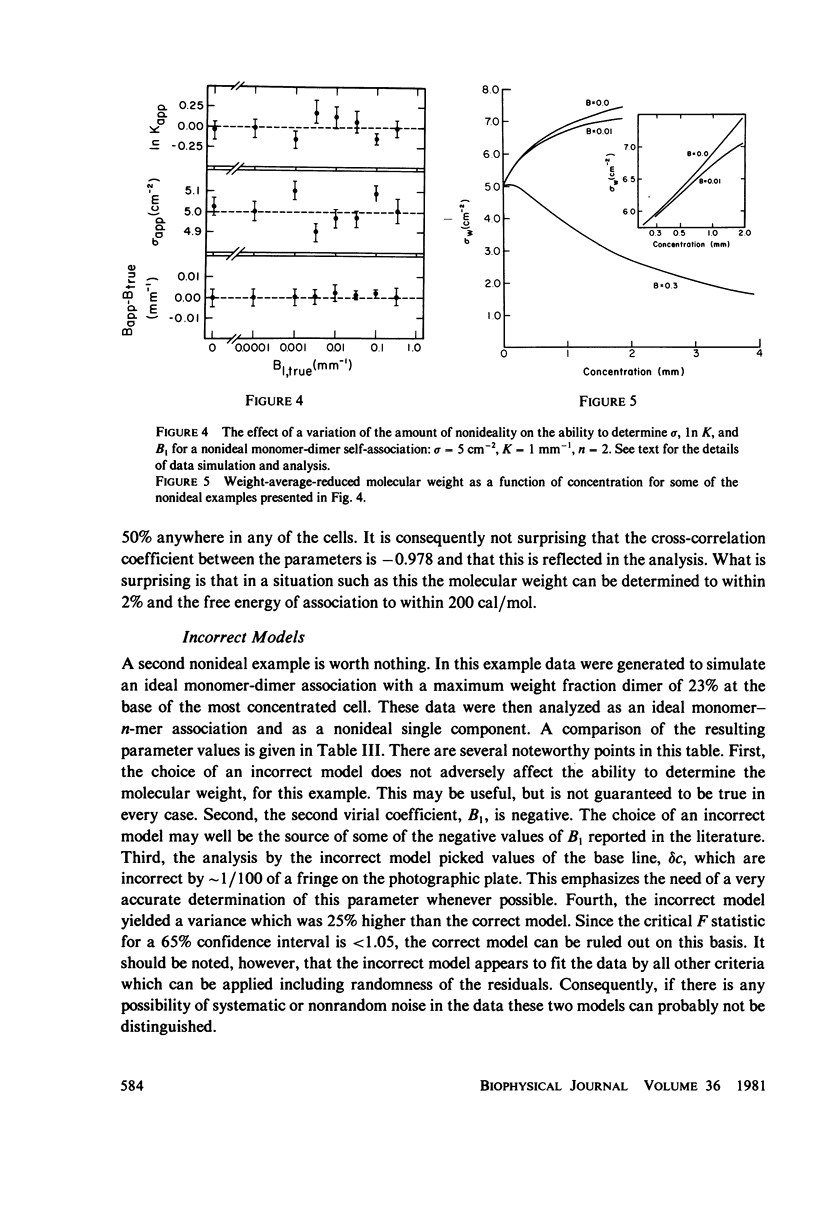


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Johnson M. L., Mills F. C., Halvorson H. R., Shapiro S. The linkage between oxygenation and subunit dissociation in human hemoglobin. Consequences for the analysis of oxygenation curves. Biochemistry. 1975 Nov 18;14(23):5128–5134. doi: 10.1021/bi00694a017. [DOI] [PubMed] [Google Scholar]
- Ansevin A. T., Roark D. E., Yphantis D. A. Improved ultracentrifuge cells for high-speed sedimentation equilibrium studies with interference optics. Anal Biochem. 1970 Mar;34:237–261. doi: 10.1016/0003-2697(70)90103-x. [DOI] [PubMed] [Google Scholar]
- Davies P. J., Wallach D., Willingham M., Pastan I., Lewis M. S. Self-association of chicken gizzard filamin and heavy merofilamin. Biochemistry. 1980 Apr 1;19(7):1366–1372. doi: 10.1021/bi00548a015. [DOI] [PubMed] [Google Scholar]
- Gladner J. A., Lewis M. S., Chung S. I. Molecular properties of lamprey fibrinogen. J Biol Chem. 1981 Feb 25;256(4):1772–1781. [PubMed] [Google Scholar]
- Haschemeyer R. H., Bowers W. F. Exponential analysis of concentration or concentration difference data for discrete molecular weight distributions in sedimentation equilibrium. Biochemistry. 1970 Jan 20;9(2):435–445. doi: 10.1021/bi00804a035. [DOI] [PubMed] [Google Scholar]
- Holladay L. A., Sohpianolpoulos A. J. Nonideal associating systems. I. Documentation of a new method for determining the parameters from sedimentation equilibrium data. J Biol Chem. 1972 Jan 25;247(2):427–439. [PubMed] [Google Scholar]
- Johnson M. L., Halvorson H. R., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: analysis of linkage functions for constituent energy terms. Biochemistry. 1976 Nov 30;15(24):5363–5371. doi: 10.1021/bi00669a024. [DOI] [PubMed] [Google Scholar]
- Johnson M. L., Yphantis D. A. Subunit association and heterogeneity of Limulus polyphemus hemocyanin. Biochemistry. 1978 Apr 18;17(8):1448–1455. doi: 10.1021/bi00601a014. [DOI] [PubMed] [Google Scholar]
- Kar E. G., Aune K. C. Analyses of sedimentation equilibrium data. Anal Biochem. 1974 Nov;62(1):1–18. doi: 10.1016/0003-2697(74)90365-0. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Knott G. D. Simulation studies of self-associating systems; discrimination between specific and isodesmic associations. Biophys Chem. 1976 Jul;5(1-2):171–183. doi: 10.1016/0301-4622(76)80033-6. [DOI] [PubMed] [Google Scholar]
- Milthorpe B. K., Jeffrey P. D., Nichol L. W. The direct analysis of sedimentation equilibrium results obtained with polymerizing systems. Biophys Chem. 1975 Apr;3(2):169–176. doi: 10.1016/0301-4622(75)80007-x. [DOI] [PubMed] [Google Scholar]
- Roark D. E., Yphantis D. A. Studies of self-associating systems by equilibrium ultracentrifugation. Ann N Y Acad Sci. 1969 Nov 7;164(1):245–278. doi: 10.1111/j.1749-6632.1969.tb14043.x. [DOI] [PubMed] [Google Scholar]
- Stafford W. F., 3rd Graphical analysis of nonideal monomer N-mer, isodesmic, and type II indefinite self-associating systems by equilibrium ultracentrifugation. Biophys J. 1980 Jan;29(1):149–166. doi: 10.1016/S0006-3495(80)85122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Yphantis D. A. Virial expansions for ideal self-associating systems. Biophys J. 1972 Oct;12(10):1359–1365. doi: 10.1016/S0006-3495(72)86167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuchet S. Equilibrium centrifugation of proteins in acid solutions. Ovalbumin in aqueous acetic, propionic, and butyric acids. Arch Biochem Biophys. 1976 Dec;177(2):437–460. doi: 10.1016/0003-9861(76)90456-2. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Yphantis D. A. Equilibrium centrifugation of proteins in acidic solutions. Beta-lactoglobulin A in aqueous acetic, propionic, and butyric acids. Arch Biochem Biophys. 1976 Apr;173(2):495–516. doi: 10.1016/0003-9861(76)90287-3. [DOI] [PubMed] [Google Scholar]
- Tang L. H., Powell D. R., Escott B. M., Adams E. T., Jr Analysis of various indefinite self-associations. Biophys Chem. 1977 Sep;7(2):121–139. doi: 10.1016/0301-4622(77)80005-7. [DOI] [PubMed] [Google Scholar]
- Wan P. J., Adams E. T., Jr Molecular weights and molecular-weight distributions from ultracentrifugation of nonideal solutions. Biophys Chem. 1976 Jul;5(1-2):207–241. doi: 10.1016/0301-4622(76)80036-1. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


