Abstract
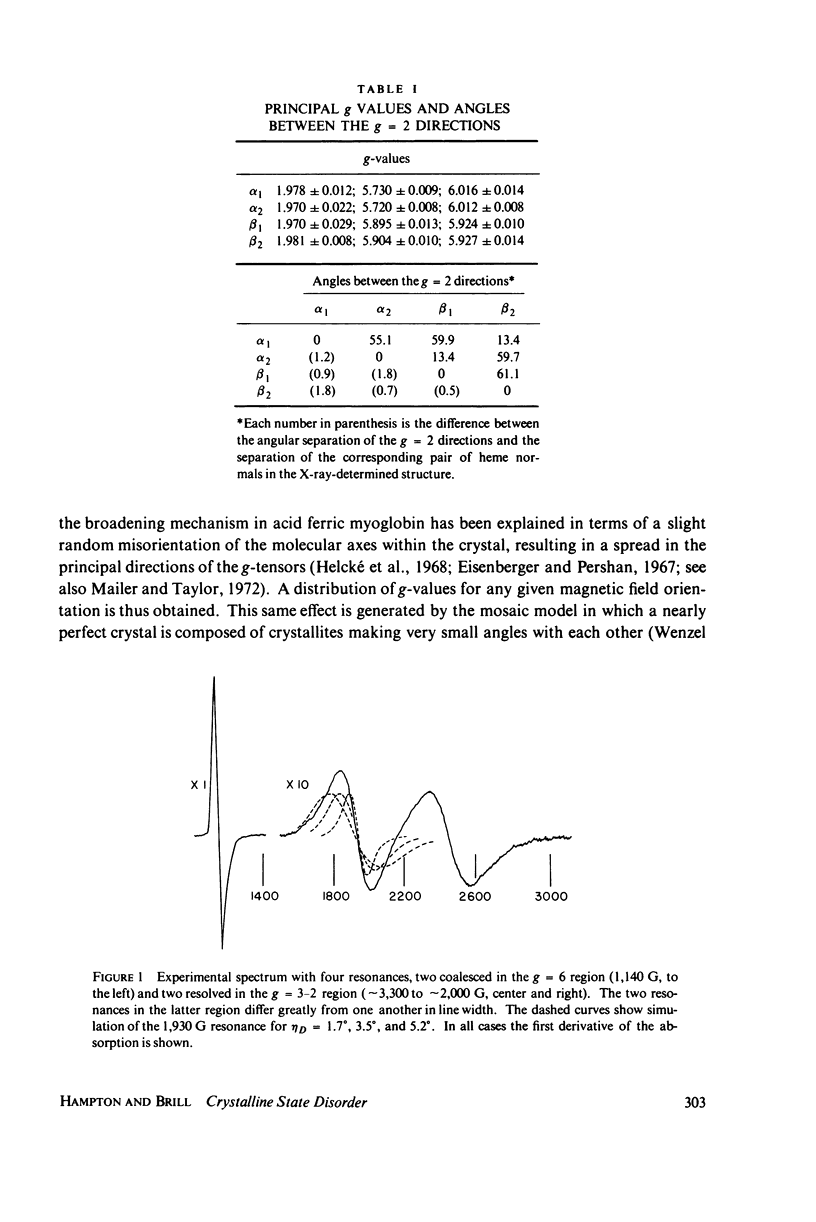
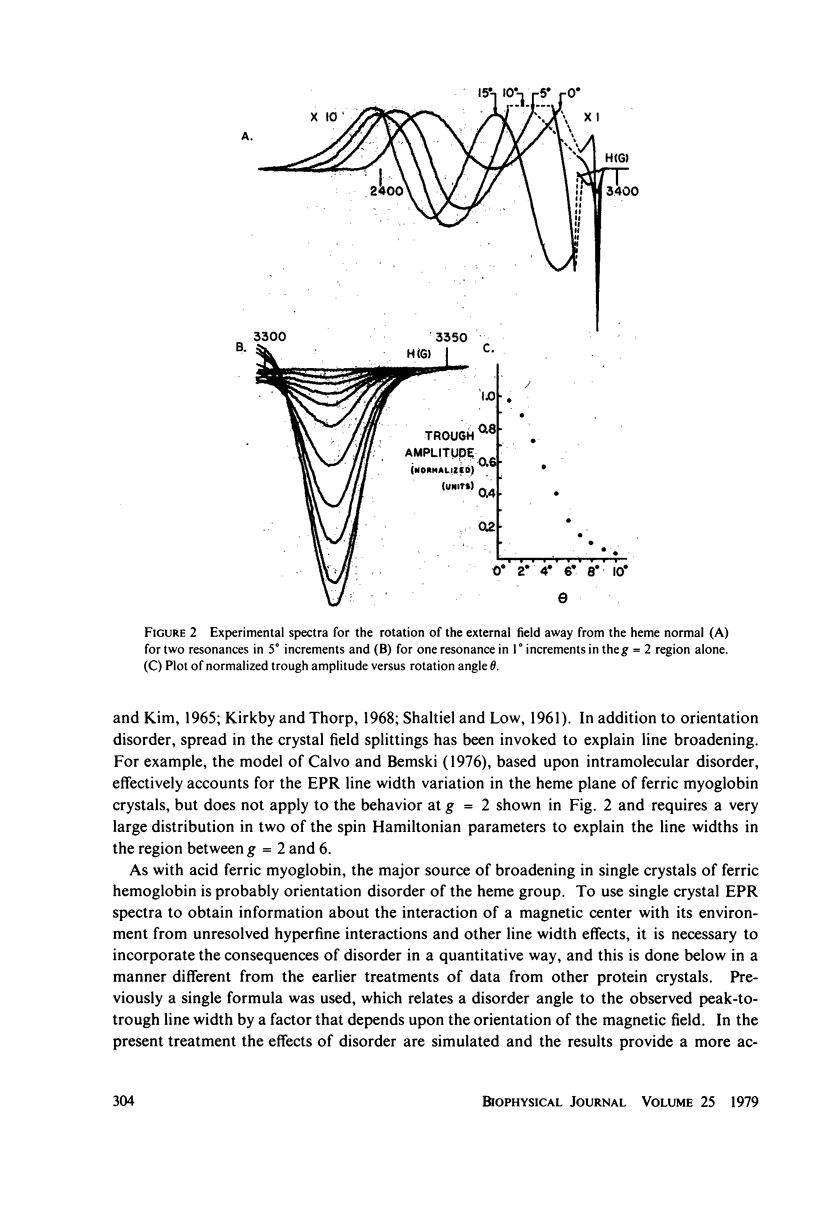
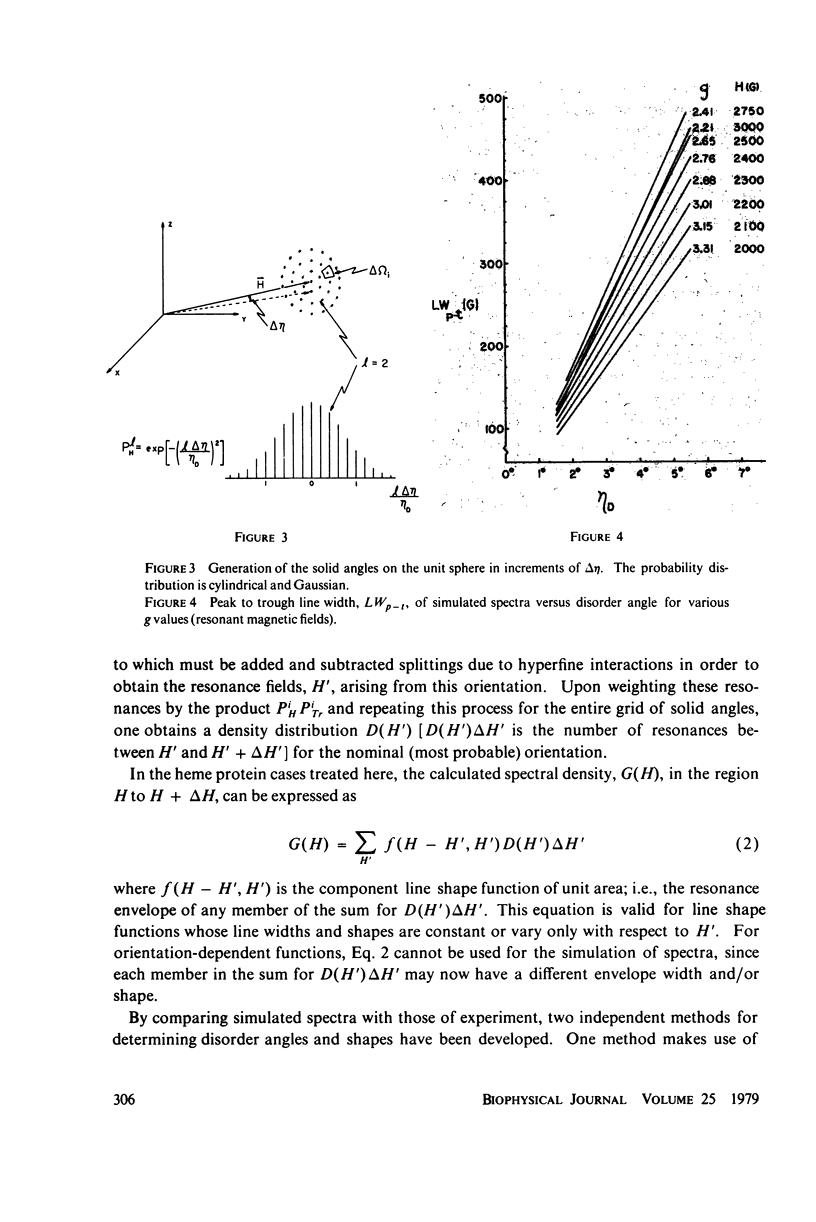
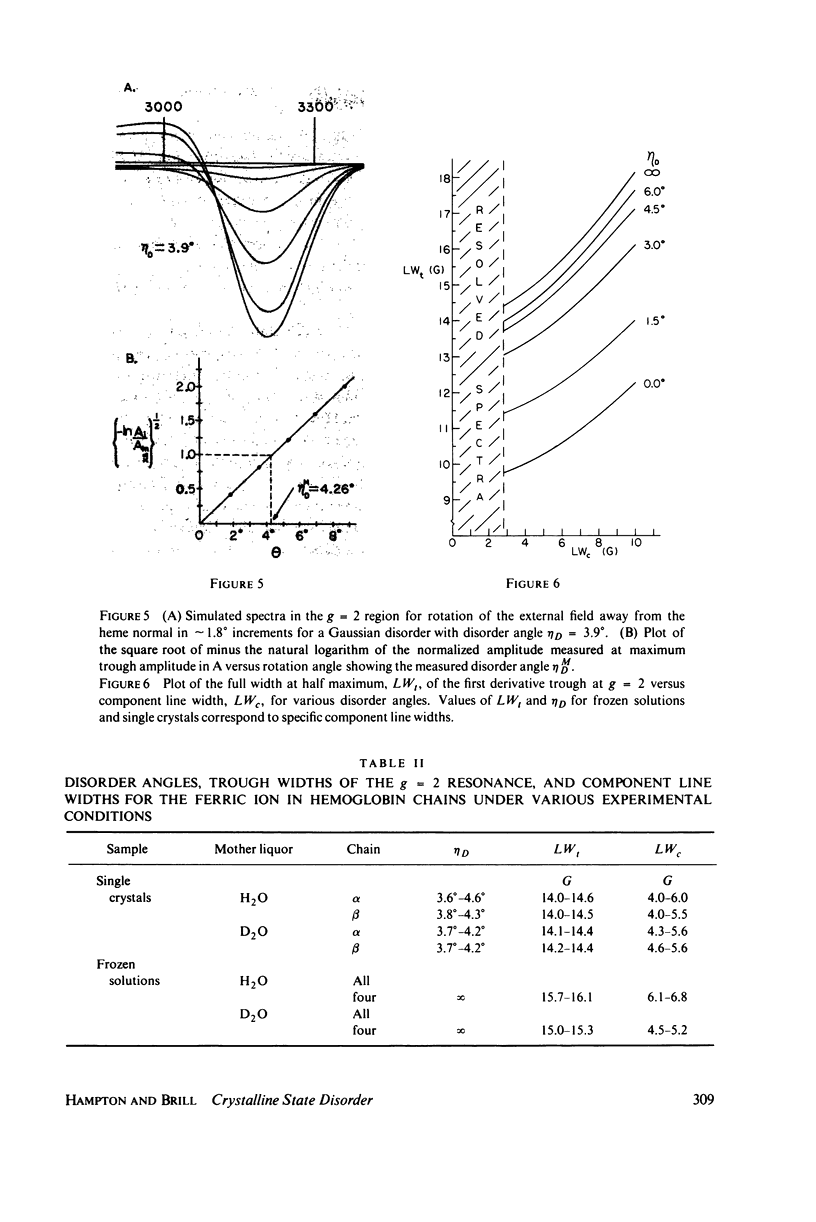
In X-band electron paramagnetic resonance spectra from single crystals of horse ferric hemoglobin, observed line widths at the low- and high-field extrema are 30 and 24 g, and as much as 400 G in the intermediate region. This behavior is similar to that of ferric myoglobin. Due to large anisotropy in the g-tensors, the line width variation can be accounted for on the basis of heme orientation disorder. This disorder is characterized by an angle, determined here by two independent methods. In these computations Gaussian disorder on a sphere is assumed. The disorder angle is found to be constant on the sphere and about 4 degrees for both alpha- and beta- chains. Treatment of crystals with heavy water (buffer) increases the disorder. Since ligand nitrogen hyperfine couplings are available from hemoglobin electron nuclear double resonance, single crystal electron paramagnetic resonance spectra can be simulated by superimposing hyperfine bands, where the line width of the component bands is a variable and the disorder model above is employed. Comparison with observed resonances fixes the hyperfine component line widths. These component line widths from ferric hemoglobin in the crystalline state are found to be smaller than those in frozen solution.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRILL A. S., VENABLE J. H., Jr ELECTRON PARAMAGNETIC RESONANCE IN SINGLE CRYSTALS OF CUPRIC INSULIN. Nature. 1964 Aug 15;203:752–754. doi: 10.1038/203752a0. [DOI] [PubMed] [Google Scholar]
- Brill A. S., Hampton D. A. Quantitative evaluation of contributions to electron paramagnetic resonance line widths in ferric hemoglobin single crystals. Biophys J. 1979 Feb;25(2 Pt 1):313–322. doi: 10.1016/s0006-3495(79)85294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill A. S., Williams R. J. The absorption spectra, magnetic moments and the binding of iron in some haemoproteins. Biochem J. 1961 Feb;78(2):246–253. doi: 10.1042/bj0780246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger P., Pershan P. S. Magnetic resonance studies of met-myoglobin and myoglobin azide. J Chem Phys. 1967 Nov 1;47(9):3327–3333. doi: 10.1063/1.1712394. [DOI] [PubMed] [Google Scholar]
- Feher G., Isaacson R. A., Scholes C. P., Nagel R. Electron nuclear double resonance (ENDOR) investigation on myoglobin and hemoglobin. Ann N Y Acad Sci. 1973 Dec 31;222:86–101. doi: 10.1111/j.1749-6632.1973.tb15254.x. [DOI] [PubMed] [Google Scholar]
- Helcké G. A., Ingram D. J., Slade E. F. Electron resonance studies of haemoglobin derivatives. 3. Line-width and g-value measurements of acid-met myoglobin and of met myoglobin azide derivatives. Proc R Soc Lond B Biol Sci. 1968 Feb 27;169(1016):275–288. doi: 10.1098/rspb.1968.0011. [DOI] [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Mailer C., Taylor C. P. Electron paramagnetic resonance study of single crystals of horse heart ferricytochrome c at 4.2 degrees K. Can J Biochem. 1972 Oct;50(10):1048–1055. doi: 10.1139/o72-145. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Leigh J. S., Jr Electromagnetic properties of hemoproteins. IV. Single crystal electron paramagnetic resonance spectroscopy of hemoproteins at ambient temperature. J Biol Chem. 1971 Jul 10;246(13):4174–4177. [PubMed] [Google Scholar]
- Yonetani T., Schleyer H. Electromagnetic properties of hemoproteins. I. Electron paramagnetic resonance absorptions of single crystals of ferrimyoglobin and cytochrome c peroxidase. J Biol Chem. 1967 Sep 10;242(17):3919–3925. [PubMed] [Google Scholar]


