Abstract
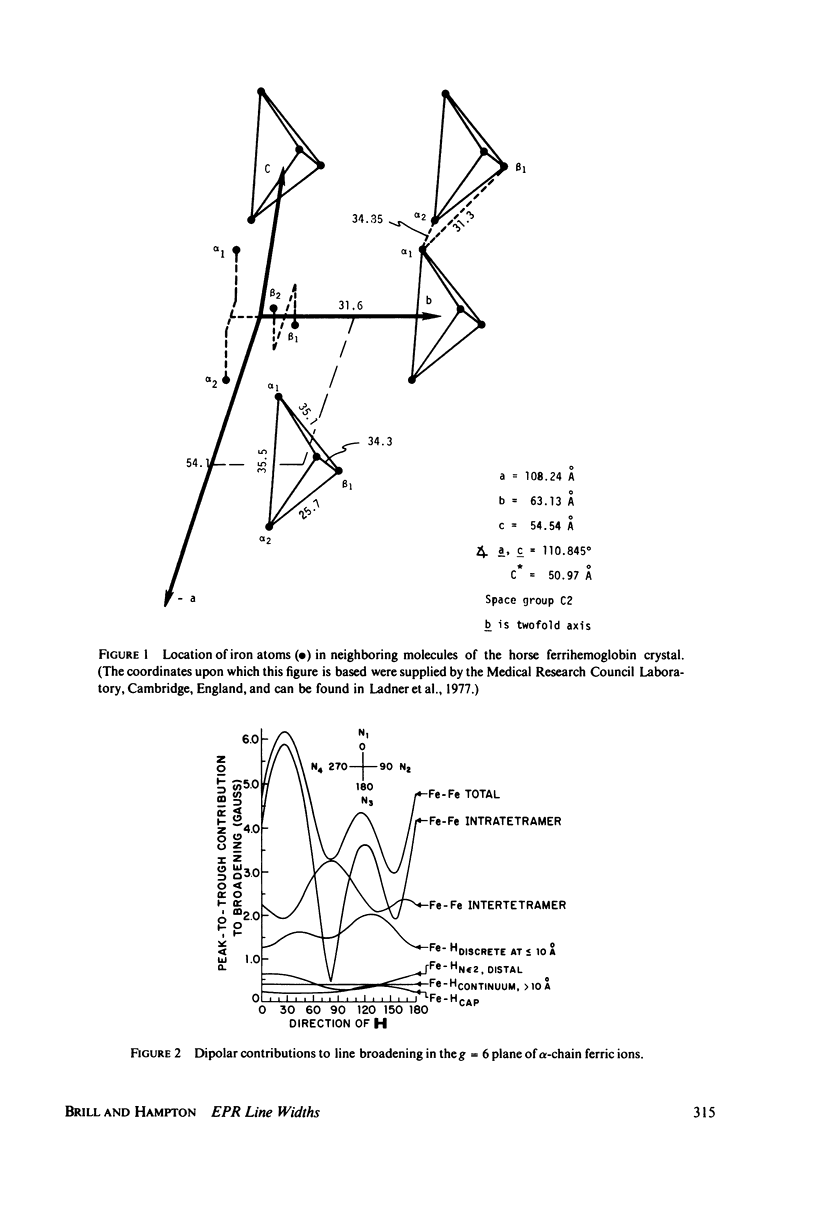
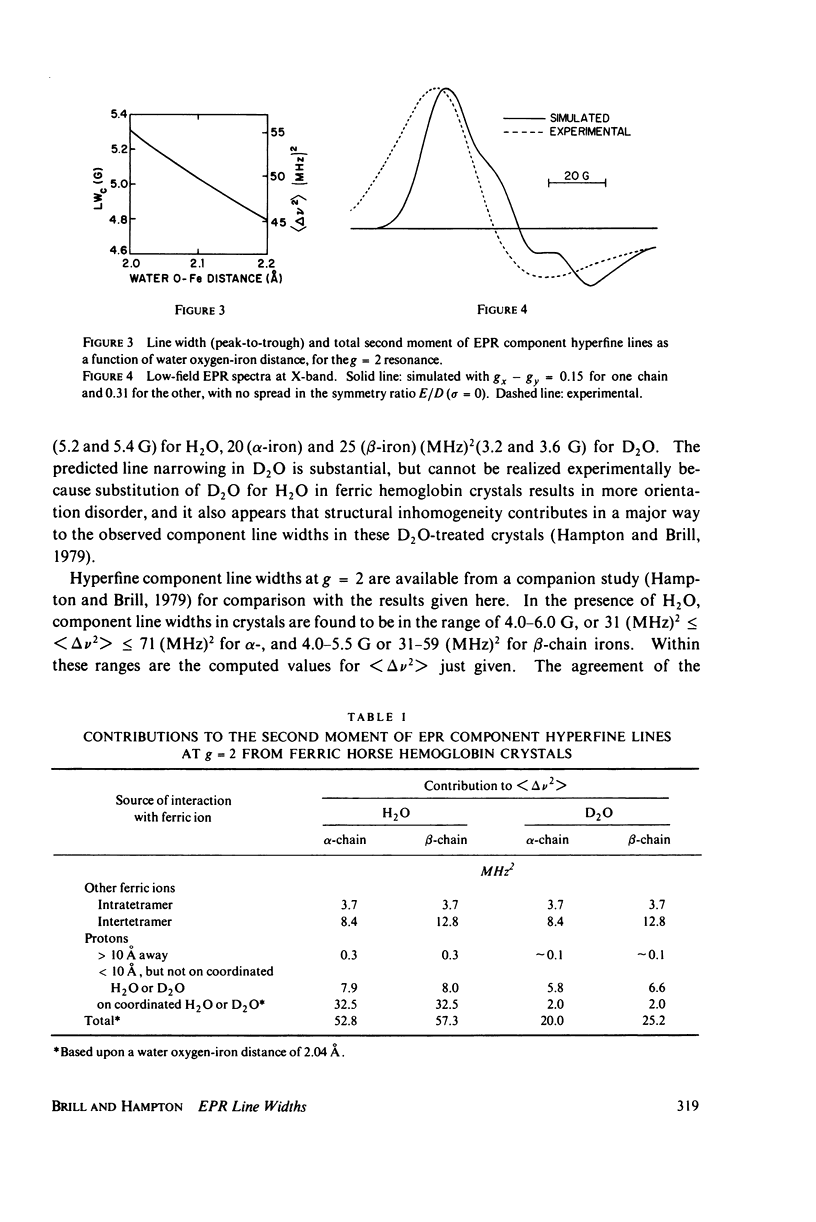
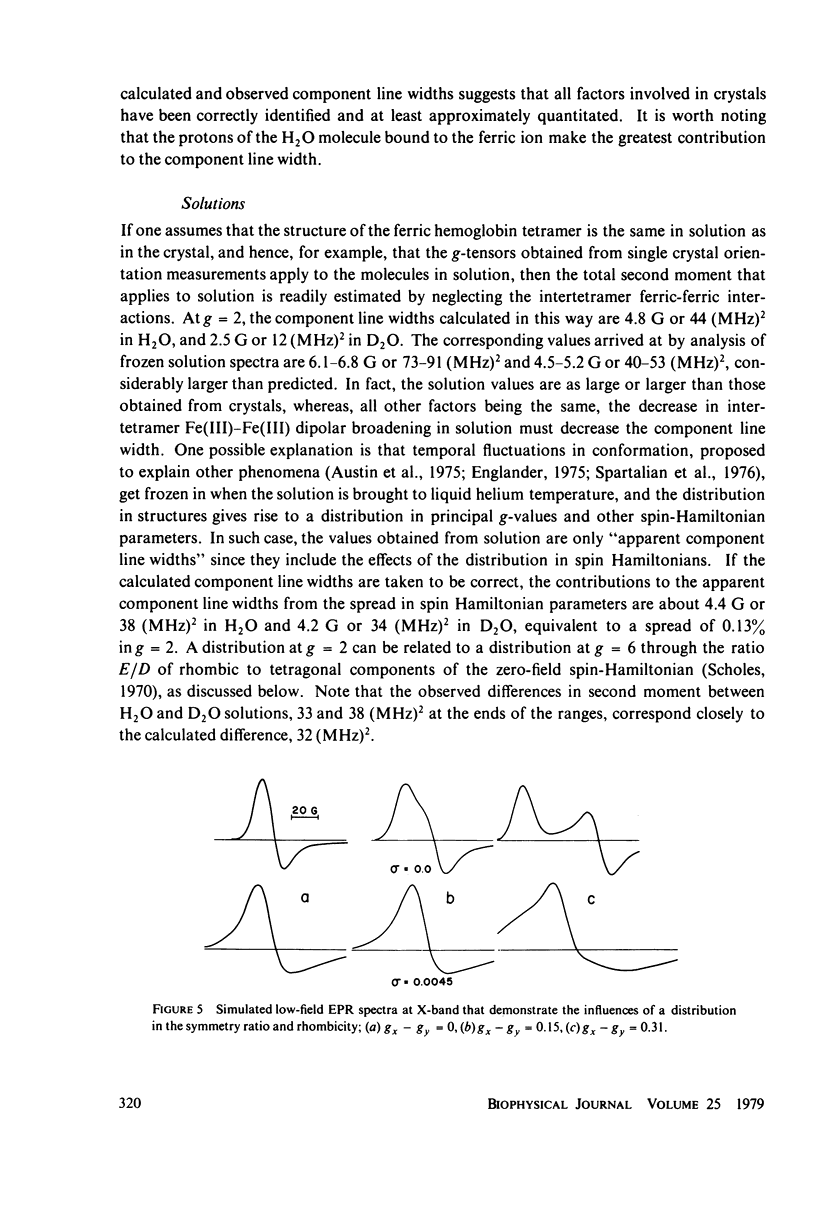
The contributions to the dipolar broadening of ferric magnetic resonances, from crystals of hemoglobin for which the atomic coordinates are known, have been calculated. The total second moment of the g = 2 resonance so determined is about 50 (MHz)2 or 5.0 G (peak-to-trough), figures consistent with the range of values found from analysis of experimental data. Two-thirds of this second moment comes from the two protons of the H2O molecule coordinated to the iron. Treatment with D2O is predicted to reduce the total second moment at g = 2 to about 25 (MHz)2, whereas the experimental measurements on single crystals show no decrease. If the structure of the tetramer is assumed to be the same when in solution as in the crystal, the total second moment is readily redetermined for hemoglobin in solution; the value so obtained is found to be significantly smaller than that from analysis of the g = 2 resonance measured in frozen solution. These two unexpected observations can be explained in terms of distributions in spin Hamiltonian parameters, the spread depending upon the nature of the sample--crystal or solution, ordinary or heavy water-treated. This distribution in H2O and D2O solutions appears to be about the same, since the measured differences in component line width agree with the calculated difference in dipolar contributions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Englander S. W. Measurement of structural and free energy changes in hemoglobin by hydrogen exchange methods. Ann N Y Acad Sci. 1975 Apr 15;244:10–27. doi: 10.1111/j.1749-6632.1975.tb41518.x. [DOI] [PubMed] [Google Scholar]
- Feher G., Isaacson R. A., Scholes C. P., Nagel R. Electron nuclear double resonance (ENDOR) investigation on myoglobin and hemoglobin. Ann N Y Acad Sci. 1973 Dec 31;222:86–101. doi: 10.1111/j.1749-6632.1973.tb15254.x. [DOI] [PubMed] [Google Scholar]
- Hampton D. A., Brill A. S. Crystalline state disorder and hyperfine component line widths in ferric hemoglobin chains. Biophys J. 1979 Feb;25(2 Pt 1):301–311. doi: 10.1016/s0006-3495(79)85293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Miurhead H., Cox J. M., Goaman L. C., Mathews F. S., McGandy E. L., Webb L. E. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: (1) x-ray analysis. Nature. 1968 Jul 6;219(5149):29–32. doi: 10.1038/219029a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Schoenborn B. P. A neutron diffraction analysis of myoglobin. 3. Hydrogen-deuterium bonding in side chains. Cold Spring Harb Symp Quant Biol. 1972;36:569–575. doi: 10.1101/sqb.1972.036.01.071. [DOI] [PubMed] [Google Scholar]
- Scholes C. P., Isaacson R. A., Feher G. Electron nuclear double resonance studies on heme proteins: determination of the interaction of Fe 3+ with its ligand nitrogens in metmyoglobin. Biochim Biophys Acta. 1972 Apr 15;263(2):448–452. doi: 10.1016/0005-2795(72)90098-0. [DOI] [PubMed] [Google Scholar]
- Spartalian K., Lang G., Yonetani T. Low temperature photodissociation studies of ferrous hemoglobin and myoglobin complexes by Mössbauer spectroscopy. Biochim Biophys Acta. 1976 Apr 23;428(2):281–290. doi: 10.1016/0304-4165(76)90036-2. [DOI] [PubMed] [Google Scholar]


