Abstract
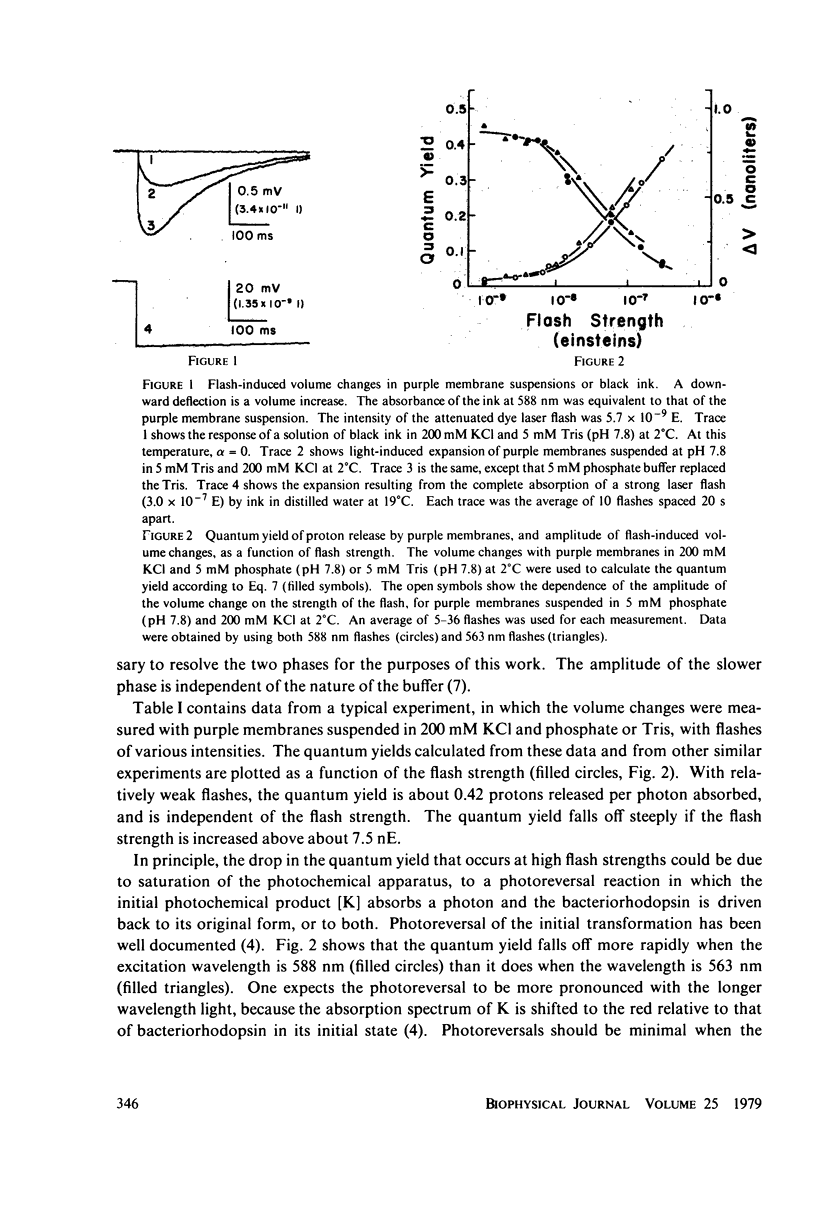
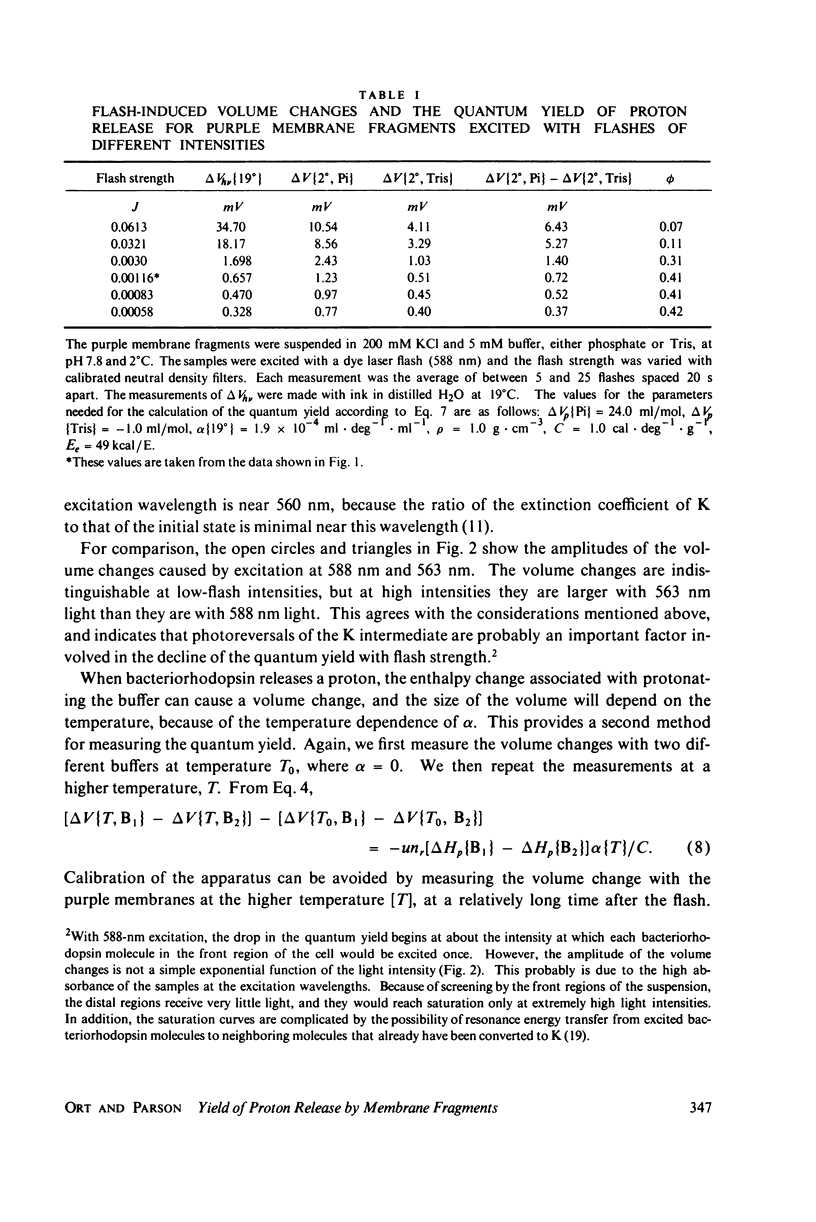
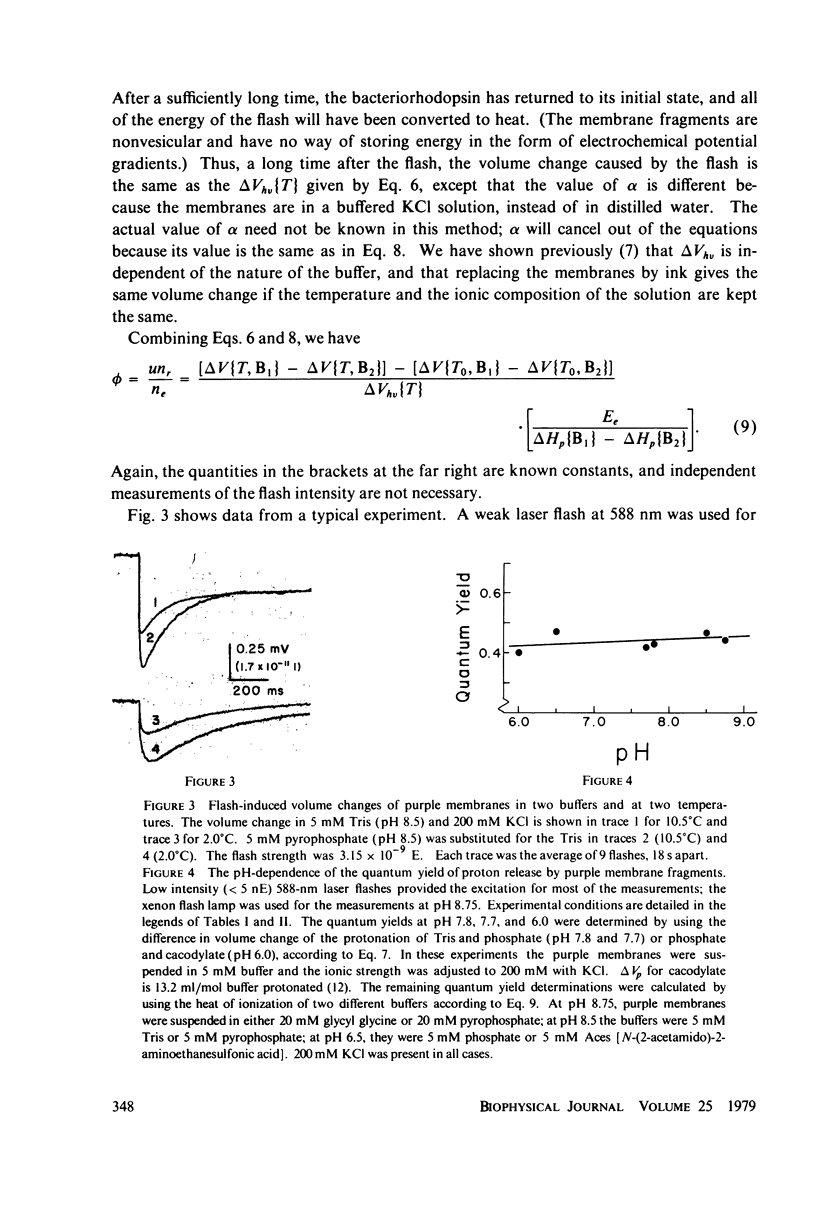
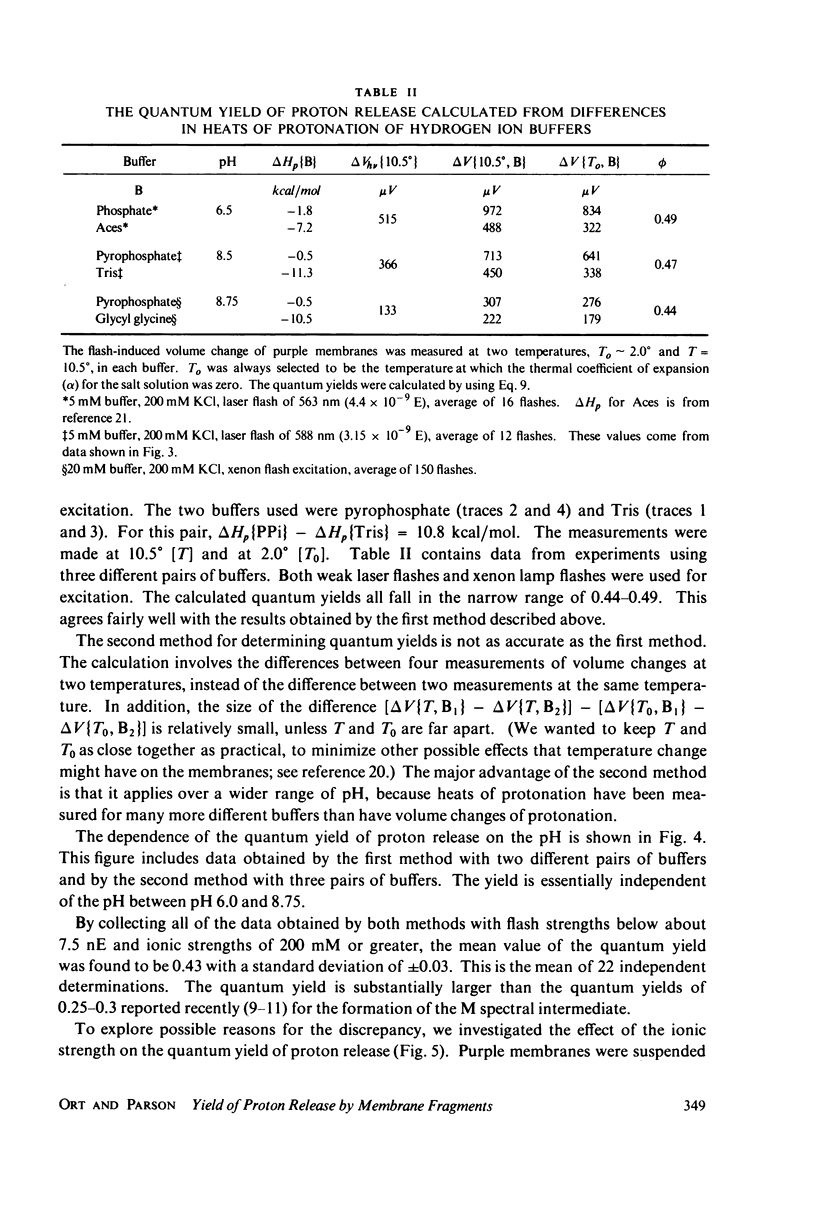
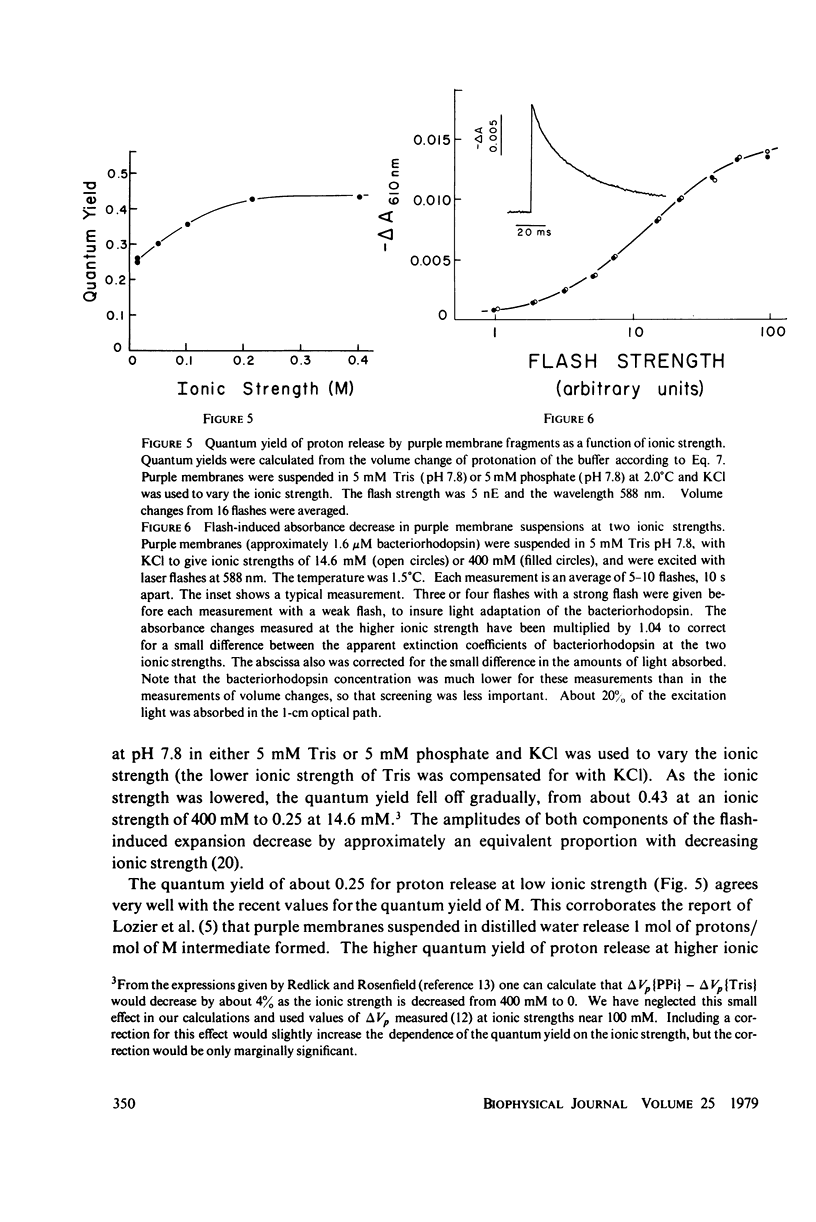
The quantum yield of proton release by bacteriorhodopsin was measured from volume changes after excitation of purple membrane fragments by short flashes. At low ionic strengths, about 0.25 mol of protons is released per einstein absorbed. This agrees well with quantum yields reported recently for the conversion of bacteriorhodopsin into a metastable state (M) that absorbs near 412 nm. However, the quantum yield of proton release increases gradually with increasing ionic strength; it plateaus with a value of 0.43 +/- 0.03 at ionic strengths above 200 mM. Changing the ionic strength has no detectable effect on the quantum yield of formation of the M spectral state. It thus appears that as many as two protons can be released and rebound in each photochemical cycle at high ionic strengths. The quantum yield of proton release is essentially independent of pH over the range 6.0-8.75. The quantum yield decreases with increasing flash strength, apparently due to photoreversal of the initial photochemical reaction.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Ebrey T. G. The quantum efficiency for the photochemical conversion of the purple membrane protein. Biophys J. 1977 Feb;17(2):185–191. doi: 10.1016/S0006-3495(77)85636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N., Wilms M. Flash photometric experiments on the photochemical cycle of bacteriorhodopsin. Biophys Struct Mech. 1975 May 30;1(3):259–271. doi: 10.1007/BF00535760. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Becher B., Mao B., Kilbride P., Honig B. Exciton interactions and chromophore orientation in the purple membrane. J Mol Biol. 1977 May 25;112(3):377–397. doi: 10.1016/s0022-2836(77)80188-5. [DOI] [PubMed] [Google Scholar]
- Goldschmidt C. R., Kalisky O., Rosenfeld T., Ottolenghi M. The quantum efficiency of the bacteriorhodopsin photocycle. Biophys J. 1977 Feb;17(2):179–183. doi: 10.1016/S0006-3495(77)85635-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt C. R., Ottolenghi M., Korenstein R. On the primary quantum yields in the bacteriorhodopsin photocycle. Biophys J. 1976 Jul;16(7):839–843. doi: 10.1016/S0006-3495(76)85732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R., Sickinger H. D., Oesterhelt D. Quantitative aspects of energy conversion in halobacteria. FEBS Lett. 1977 Oct 1;82(1):1–6. doi: 10.1016/0014-5793(77)80873-9. [DOI] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W. The photochemical cycle of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1805–1809. [PubMed] [Google Scholar]
- Oesterhelt D., Meentzen M., Schuhmann L. Reversible dissociation of the purple complex in bacteriorhodopsin and identification of 13-cis and all-trans-retinal as its chromophores. Eur J Biochem. 1973 Dec 17;40(2):453–463. doi: 10.1111/j.1432-1033.1973.tb03214.x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. Enthalpy changes during the photochemical cycle of bacteriorhodopsin. Biophys J. 1979 Feb;25(2 Pt 1):355–364. doi: 10.1016/s0006-3495(79)85297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort D. R., Parson W. W. Flash-induced volume changes of bacteriorhodopsin-containing membrane fragments and their relationship to proton movements and absorbance transients. J Biol Chem. 1978 Sep 10;253(17):6158–6164. [PubMed] [Google Scholar]
- Papadopoulos G. K., Hsiao T. L., Cassim J. Y. Determination of the retinal/protein molar ratios for the purple membranes of Halobacterium halobium and Halobacterium cutirubrum. Biochem Biophys Res Commun. 1978 Mar 15;81(1):127–132. doi: 10.1016/0006-291x(78)91639-x. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]


