Abstract
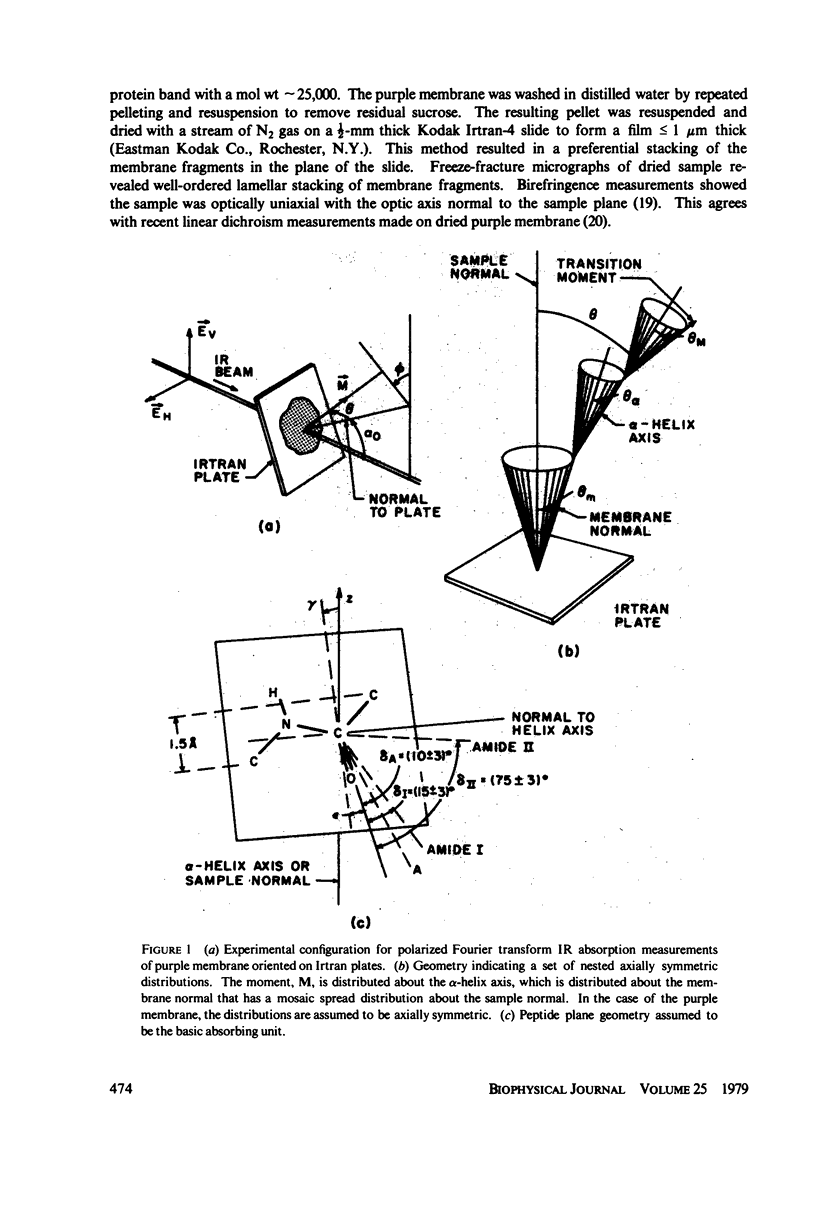
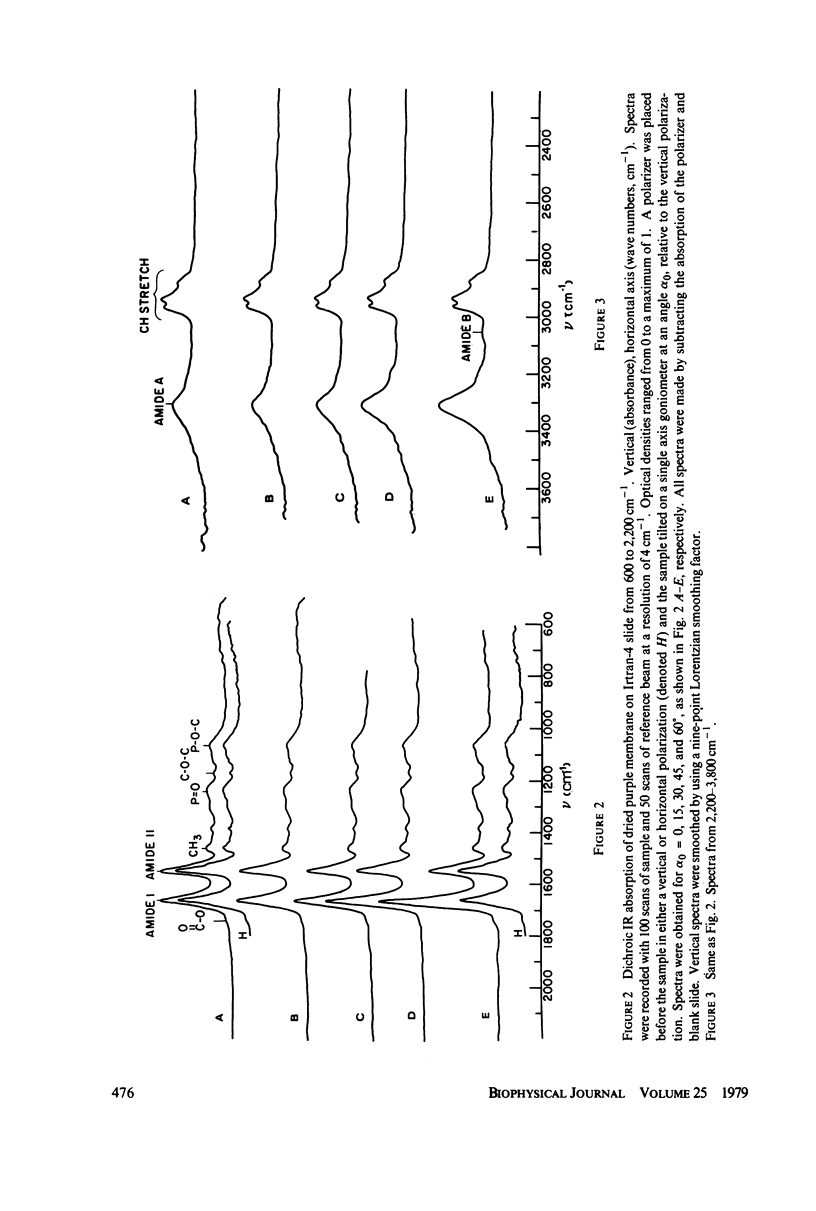
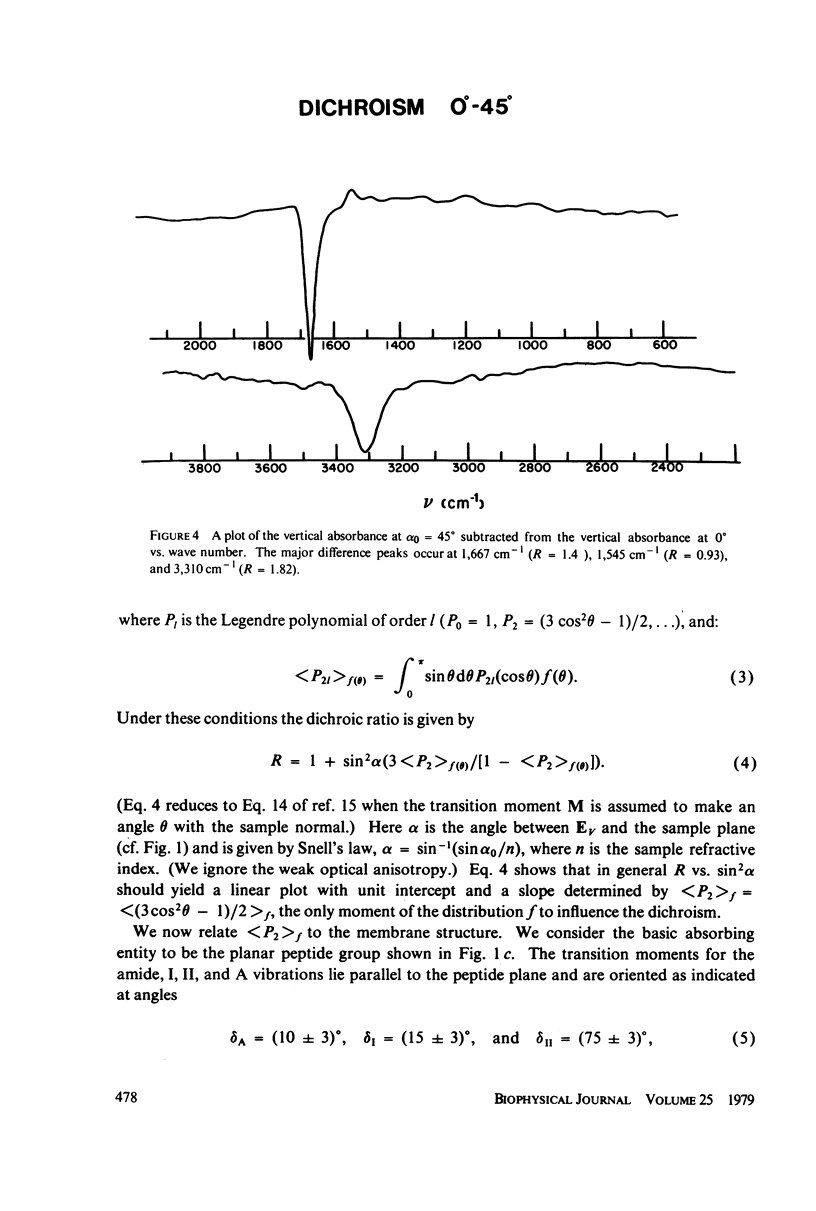
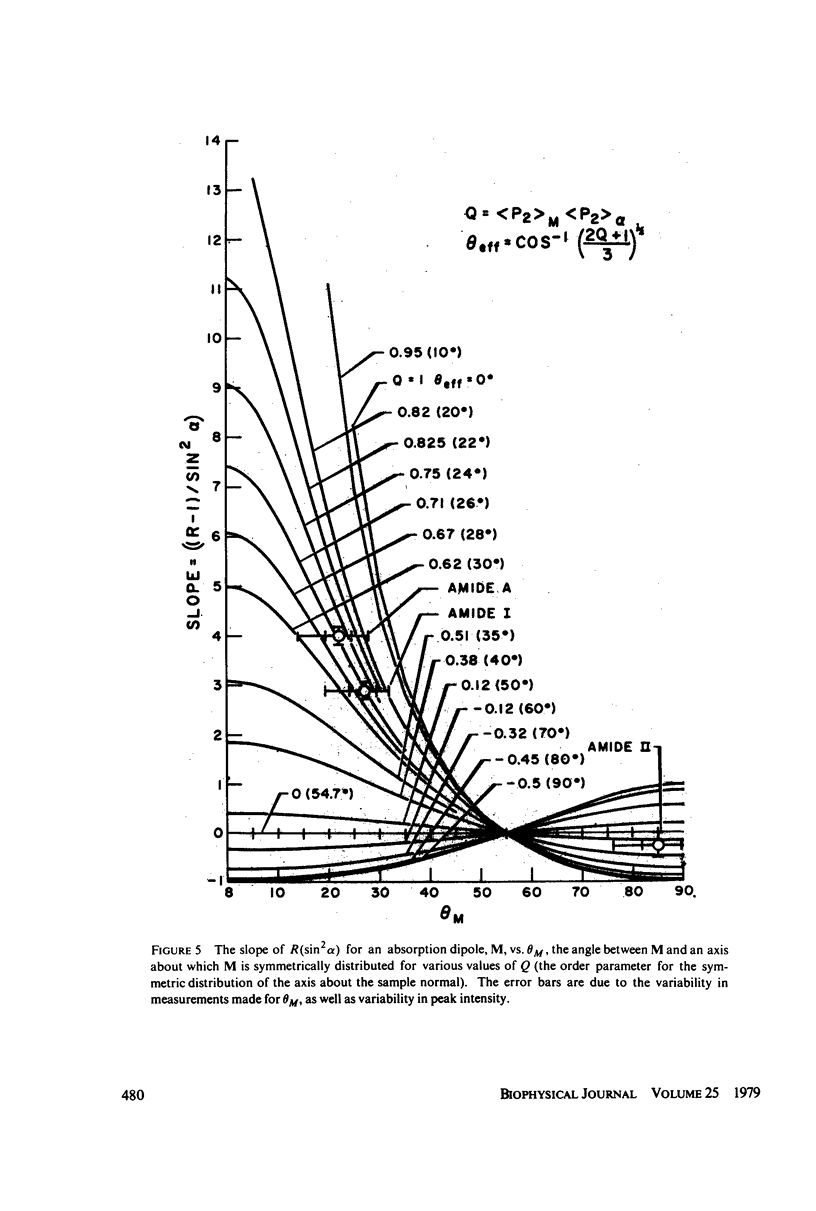
Polarized Fourier transform infrared spectroscopy has been used to study the structure of purple membrane from Halobacterium halobium. Membranes were oriented by drying a suspension of membrane fragments onto Irtran-4 slides. Dichroism measurements of the amide I, II and A peaks were used to find the average spatial orientation of the bacteriorhodopsin alpha-helices. By deriving a function that relates the observed dichroism to the orientational order parameters for the peptide groups, helical axis distribution, and mosaic spread of the membranes, the average orientation of the alpha-helices was found to lie in a range of less than 26 degrees away from the membrane normal, agreeing with electron microscopic measurements. The frequency of the amide I and A peaks is at least 10 cm-1 higher than values found for most alpha-helical polypeptides and proteins. This may indicate that bacteriorhodopsin contains distorted alpha-helical conformations.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akutsu H., Kyogoku Y. Conformational analysis of phosphatidylethanol-amine in multilayers by infrared dichroism. Chem Phys Lipids. 1975 Nov;15(2):222–242. doi: 10.1016/0009-3084(75)90045-6. [DOI] [PubMed] [Google Scholar]
- Becher B. M., Cassim J. Y. Improved isolation procedures for the purple membrane of Halobacterium halobium. Prep Biochem. 1975;5(2):161–178. doi: 10.1080/00327487508061568. [DOI] [PubMed] [Google Scholar]
- Becher B., Cassim J. Y. Effects of light adaptation on the purple membrane structure of Halobacterium halobium. Biophys J. 1976 Oct;16(10):1183–1200. doi: 10.1016/S0006-3495(76)85767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E. Bacteriorhodospin: a trans-membrane pump containing alpha-helix. J Mol Biol. 1975 Apr 5;93(2):139–158. doi: 10.1016/0022-2836(75)90124-2. [DOI] [PubMed] [Google Scholar]
- Blaurock A. E., Stoeckenius W. Structure of the purple membrane. Nat New Biol. 1971 Sep 29;233(39):152–155. doi: 10.1038/newbio233152a0. [DOI] [PubMed] [Google Scholar]
- Chapman D., Kamat V. B., Levene R. J. Infrared spectra and the chain organization of erythrocyte membranes. Science. 1968 Apr 19;160(3825):314–316. doi: 10.1126/science.160.3825.314. [DOI] [PubMed] [Google Scholar]
- Dunker A. K., Zaleske D. J. Stereochemical considerations for constructing alpha-helical protein bundles with particular application to membrane proteins. Biochem J. 1977 Apr 1;163(1):45–57. doi: 10.1042/bj1630045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz F., Lotz B., Spach G. AlphaDL and piDL helices of alternating poly-gamma-benzyl-D-L-glutamate. J Mol Biol. 1975 Feb 15;92(1):1–13. doi: 10.1016/0022-2836(75)90088-1. [DOI] [PubMed] [Google Scholar]
- Henderson R. The structure of the purple membrane from Halobacterium hallobium: analysis of the X-ray diffraction pattern. J Mol Biol. 1975 Apr 5;93(2):123–138. doi: 10.1016/0022-2836(75)90123-0. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- KRIMM S. Infrared spectra and chain conformation of proteins. J Mol Biol. 1962 Jun;4:528–540. doi: 10.1016/s0022-2836(62)80107-7. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Racker E. Light-dependent proton and rubidium translocation in membrane vesicles from Halobacterium halobium. Biochem Biophys Res Commun. 1975 Jan 2;64(3):1054–1061. doi: 10.1016/0006-291x(75)90154-0. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M. A., Lewis A. Kinetic resonance Raman spectroscopy: dynamics of deprotonation of the Schiff base of bacteriorhodopsin. Science. 1977 Mar 25;195(4284):1328–1330. doi: 10.1126/science.841330. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Andrew J. R., De Grip W. J., Stanley H. E. Opsin structure probed by raman spectroscopy of photoreceptor membranes. Science. 1976 Mar 19;191(4232):1176–1178. doi: 10.1126/science.1257742. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H. Light energy conversion in Halobacterium halobium. J Supramol Struct. 1974;2(5-6):769–774. doi: 10.1002/jss.400020519. [DOI] [PubMed] [Google Scholar]
- Susi H., Timasheff S. N., Stevens L. Infrared spectra and protein conformations in aqueous solutions. I. The amide I band in H2O and D2O solutions. J Biol Chem. 1967 Dec 10;242(23):5460–5466. [PubMed] [Google Scholar]
- Timasheff S. N., Susi H., Stevens L. Infrared spectra and protein conformations in aqueous solutions. II. Survey of globular proteins. J Biol Chem. 1967 Dec 10;242(23):5467–5473. [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Infrared spectra of plasma membrane and endoplasmic reticulum of Ehrlich ascites carcinoma. Biochim Biophys Acta. 1968 Mar 1;150(2):186–193. doi: 10.1016/0005-2736(68)90162-4. [DOI] [PubMed] [Google Scholar]


