Abstract
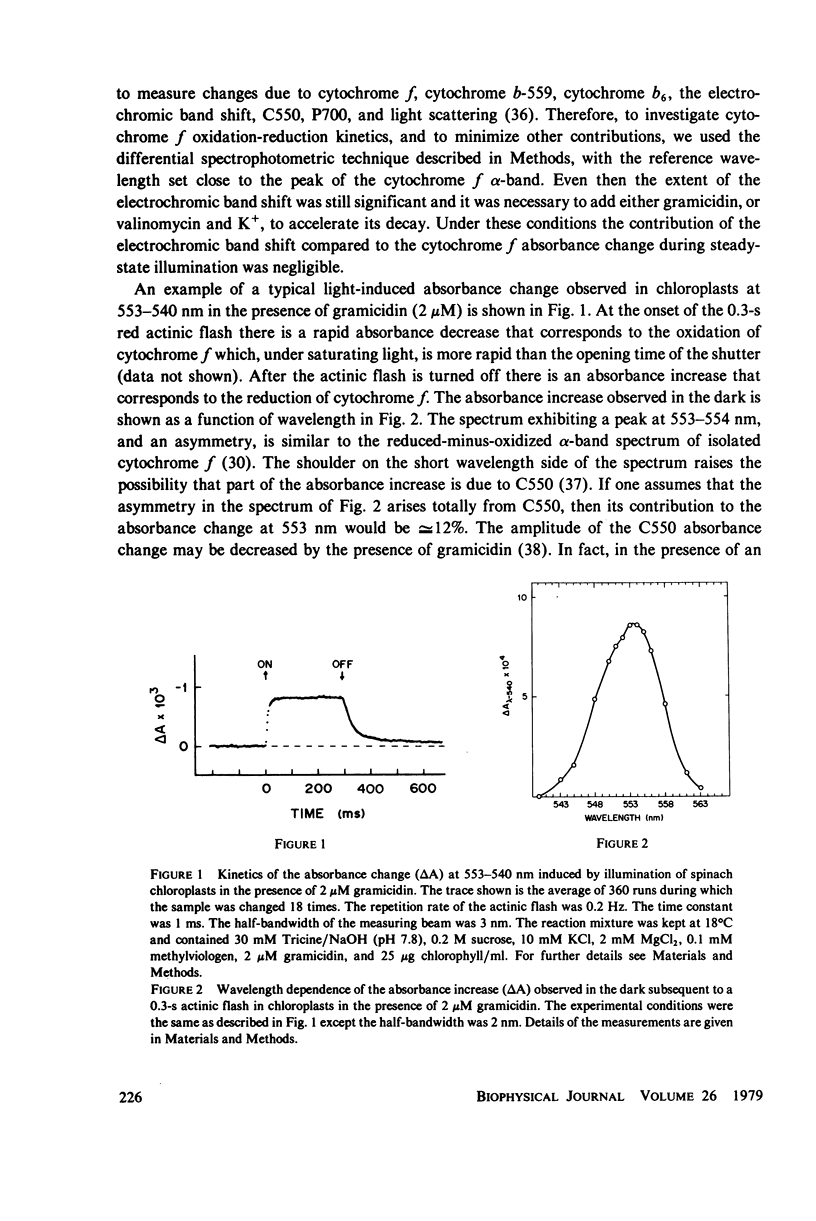
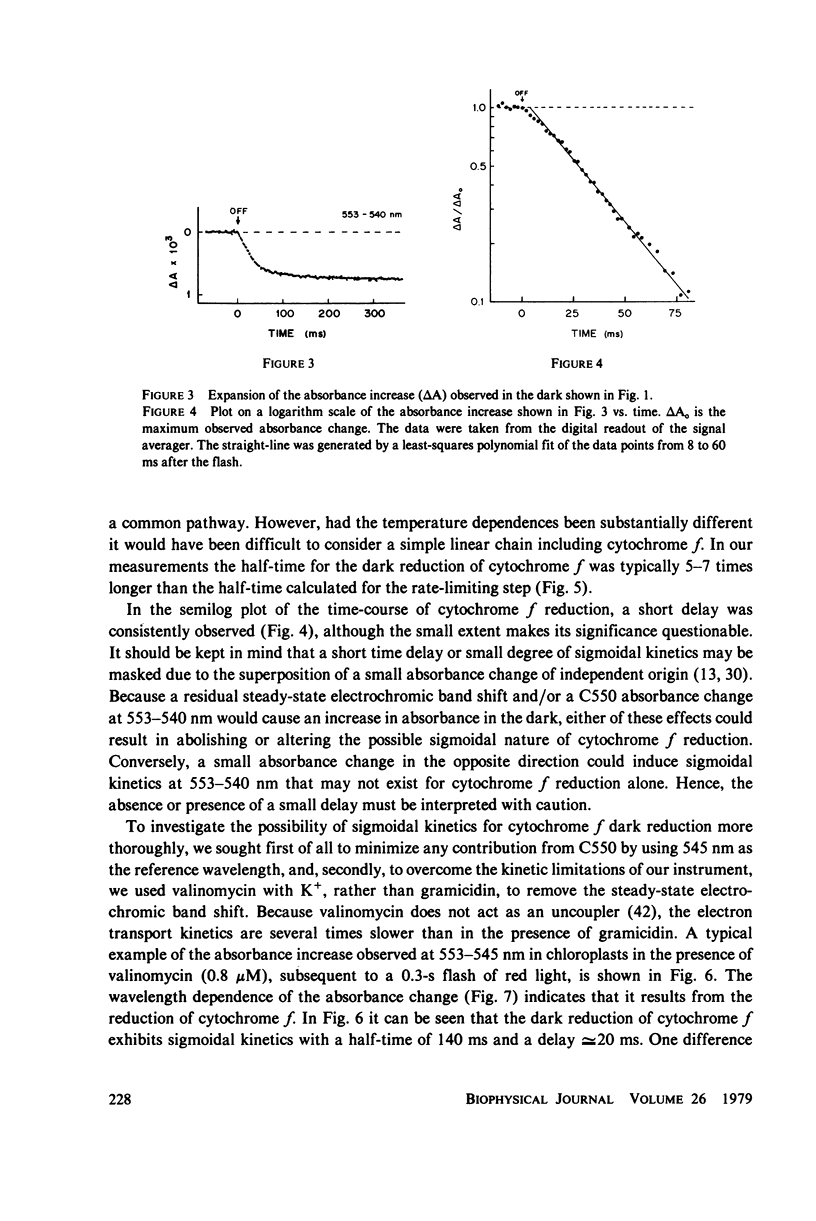
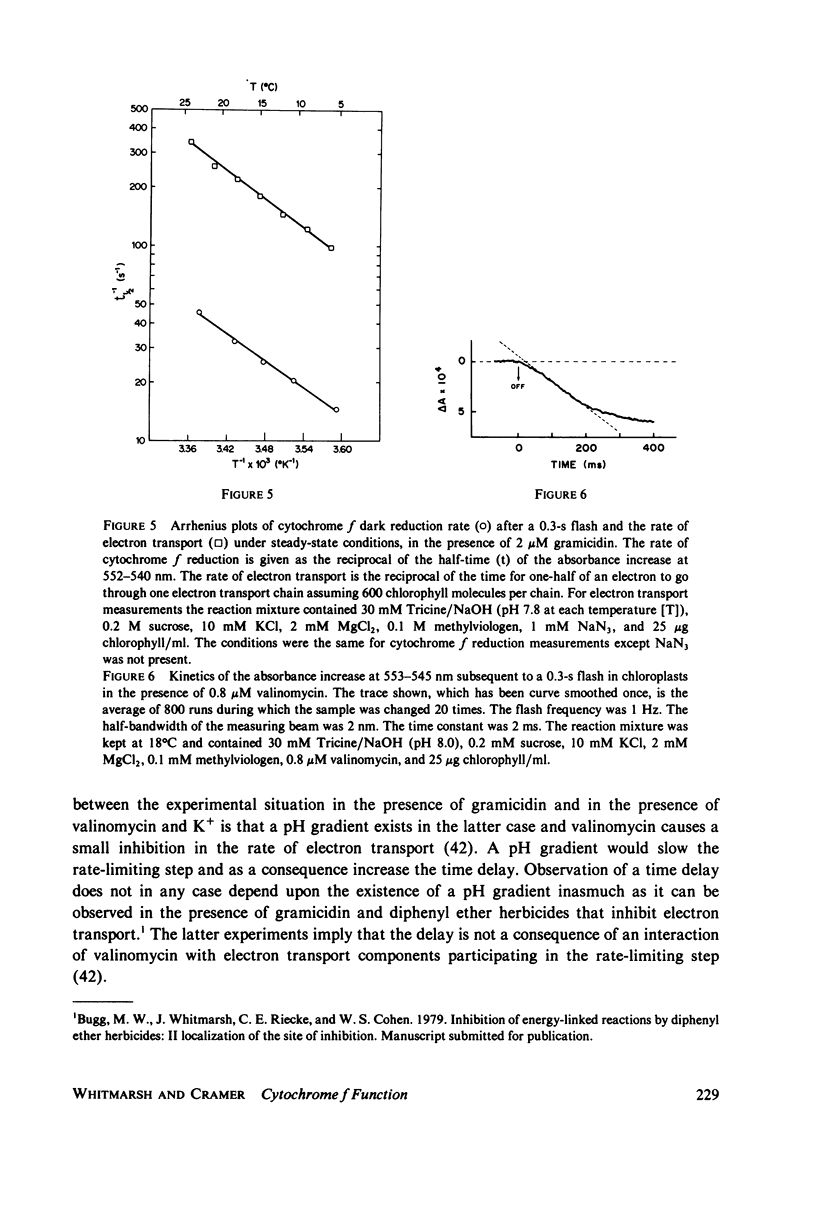
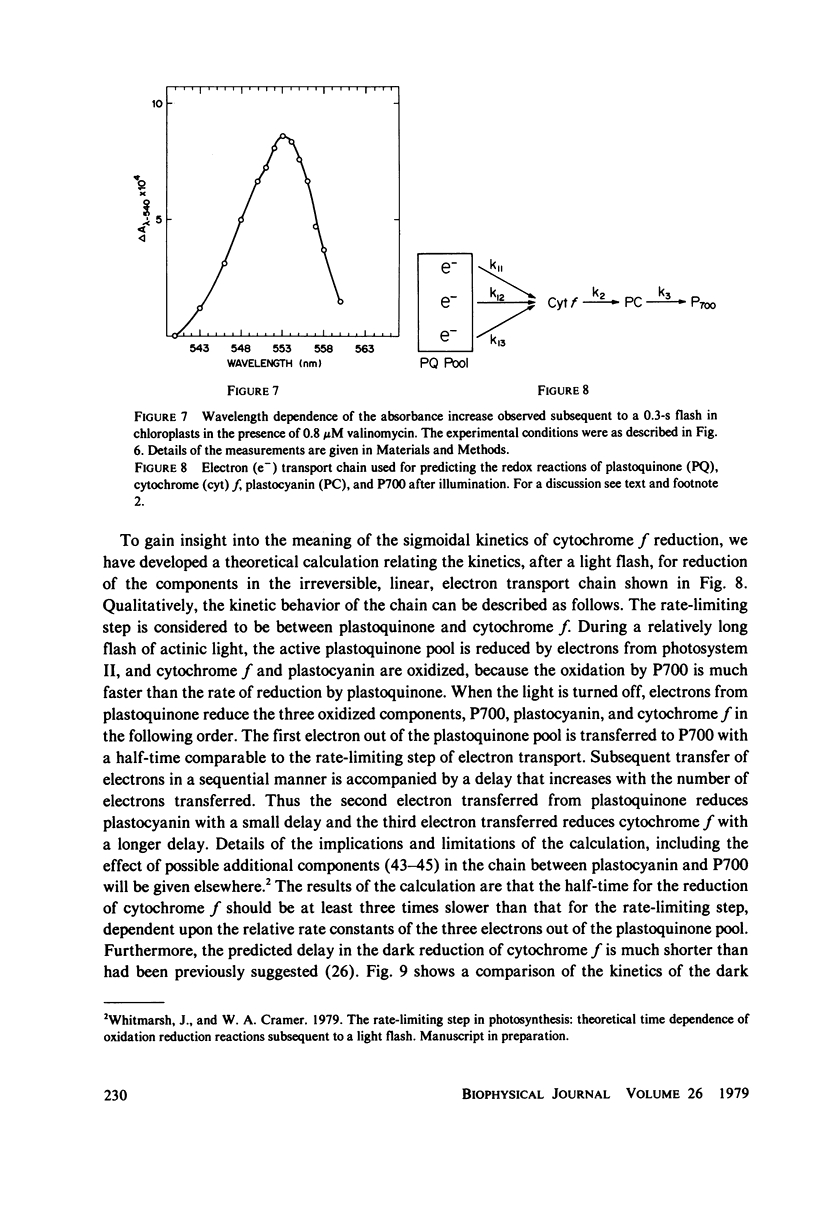
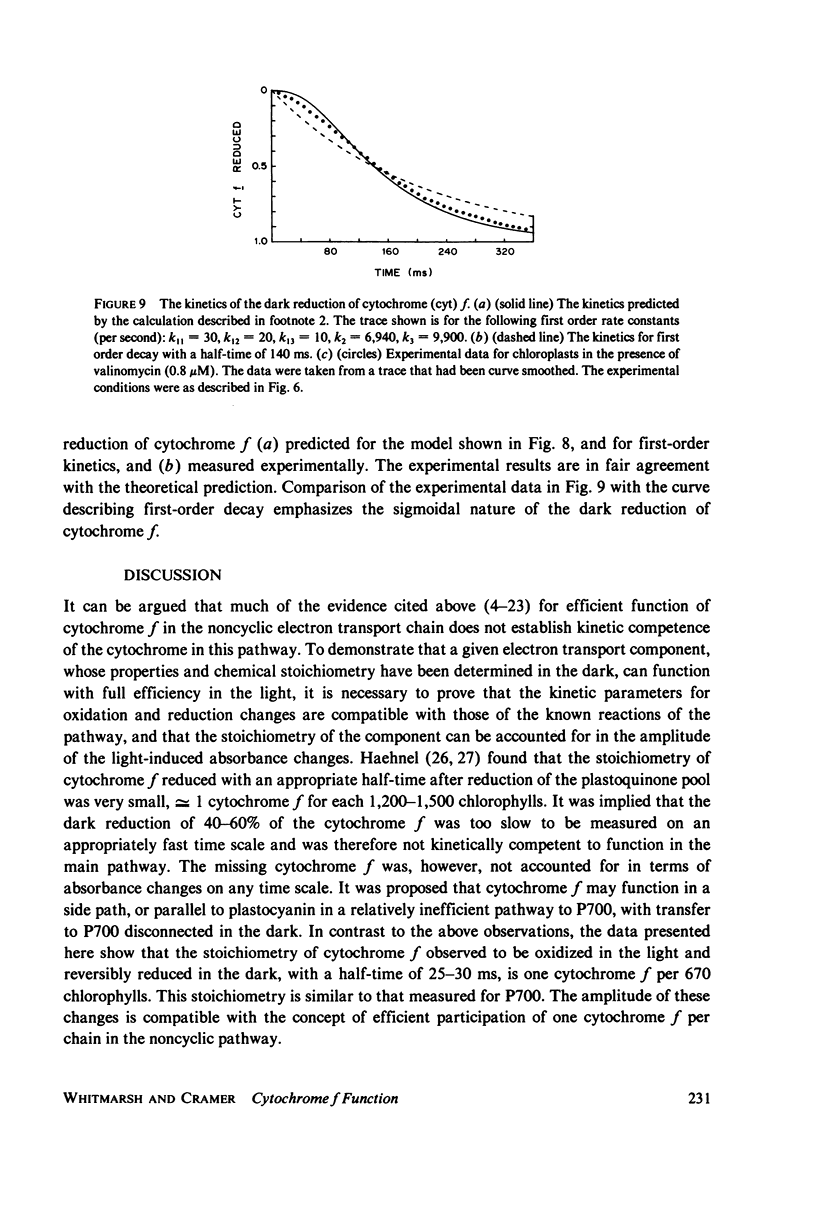
The questions of whether the stoichiometry of the turnover of cytochrome f, and the time-course of its reduction subsequent to a light flash, are consistent with efficient function in noncyclic electron transport have been investigated. Measurements were made of the absorbance change at the 553-nm alpha-band maximum relative to a reference wavelength. In the dark cytochrome f is initially fully reduced, oxidized by a 0.3-s flash, and reduced again in the dark period after the flash. In the presence of gramicidin at 18 degrees C, the dark reduction was characterized by a half-time of 25-30 ms, stoichiometries of cytochrome f:chlorophyll and P700:chlorophyll of 1:670 and 1:640, respectively, and a short time delay. The time delay in the dark reduction of cytochrome f, which is expected for a component in an intermediate position in the chain, becomes more apparent in the presence of valinomycin and K+. Under these conditions the half-time for cytochrome f dark reduction is 130-150 ms, and the delay is approximately equal to 20 ms. The measured value for the activation energy of the dark reduction of cytochrome f (11 +/- 1 kcal/mol) is the same as that for noncyclic electron transport in steady-state light. A sigmoidal time-course for the reduction of cytochrome f has been calculated for a simple linear electron transport chain. The kinetics for reduction of cytochrome f predicted by the calculation, in the presence of valinomycin and K+, are in reasonably good agreement with the experimental data. There is an appreciable amount of data in the literature to document complex properties of cytochrome f after illumination with short flashes, and evidence for complexity in a light-minus-dark transition. The data presented here, obtained after a long flash that should establish steady-state conditions, either fulfill or are consistent with the basic criteria for efficient function of cytochrome f in noncyclic electron transport.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz J., Visser J. W., van den Engh G. J., Dirks M. P. Reaction kinetics of intermediates of the photosynthetic chain between the two photosystems. Biochim Biophys Acta. 1972 Feb 28;256(2):370–380. doi: 10.1016/0005-2728(72)90067-9. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J. Kinetic behavior of cytochrome f in cyclic and noncyclic electron transport in Porphyridium cruentum. Biochemistry. 1973 Mar 13;12(6):1165–1170. doi: 10.1021/bi00730a023. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Cytochrome f and plastocyanin kinetics in Chlorella pyrenoidosa. I. Oxidation kinetics after a flash. Biochim Biophys Acta. 1977 Nov 17;462(2):362–370. doi: 10.1016/0005-2728(77)90134-7. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Cytochrome f and plastocyanin kinetics in Chlorella pyrenoidosa. II. Reduction kinetics and electric field increase in the 10 ms range. Biochim Biophys Acta. 1977 Nov 17;462(2):371–379. doi: 10.1016/0005-2728(77)90135-9. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Electron transport in photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1975 Sep 8;396(3):382–391. doi: 10.1016/0005-2728(75)90144-9. [DOI] [PubMed] [Google Scholar]
- Butler W. L. The influence of membrane potential on measurements of C-550 at room temperature. FEBS Lett. 1972 Feb 15;20(3):333–338. doi: 10.1016/0014-5793(72)80100-5. [DOI] [PubMed] [Google Scholar]
- Böhme H., Cramer W. A. Localization of a site of energy coupling between plastoquinone and cytochrome f in the electron-transport chain of spinach chloroplasts. Biochemistry. 1972 Mar 28;11(7):1155–1160. doi: 10.1021/bi00757a007. [DOI] [PubMed] [Google Scholar]
- Böhme H., Cramer W. A. Plastoquinone mediates electron transport between cytochrome b-559 and cytochrome f in spinach chloroplasts. FEBS Lett. 1971 Jul 8;15(5):349–351. doi: 10.1016/0014-5793(71)80331-9. [DOI] [PubMed] [Google Scholar]
- Cox R. P. The properties of cytochrome f and P700 in chloroplasts suspended in fluid media at sub-zero temperatures. Eur J Biochem. 1975 Jul 15;55(3):625–631. doi: 10.1111/j.1432-1033.1975.tb02200.x. [DOI] [PubMed] [Google Scholar]
- DAVENPORT H. E., HILL R. The preparation and some properties of cytochrome f. Proc R Soc Lond B Biol Sci. 1952 Apr 24;139(896):327–345. doi: 10.1098/rspb.1952.0016. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N., AMESZ J., KAMP B. M. Two photochemical systems in photosynthesis. Nature. 1961 May 6;190:510–511. doi: 10.1038/190510a0. [DOI] [PubMed] [Google Scholar]
- Delosme R., Zickler A., Joliot P. Turnover kinetics of photosystem I measured by the electrochromic effect in Chlorella. Biochim Biophys Acta. 1978 Oct 11;504(1):165–174. doi: 10.1016/0005-2728(78)90015-4. [DOI] [PubMed] [Google Scholar]
- Dolan E., Hind G. Kinetics of the reduction and oxidation of cytochromes b6 and f in isolated chloroplasts. Biochim Biophys Acta. 1974 Sep 20;357(3):380–385. doi: 10.1016/0005-2728(74)90028-0. [DOI] [PubMed] [Google Scholar]
- Givan A. L., Levine R. P. The photosynthetic electron transport chain of Chlamydomonas reinhardi. VII. Photosynthetic phosphorylation by a mutant strain of Chlamydomonas reinhardi deficient in active P700. Plant Physiol. 1967 Sep;42(9):1264–1268. doi: 10.1104/pp.42.9.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehnel W., Döring G., Witt H. T. On the reaction between chlorophyll-a, and its primary electron donors in photosynthesis. Z Naturforsch B. 1971 Nov;266(11):1171–1174. doi: 10.1515/znb-1971-1118. [DOI] [PubMed] [Google Scholar]
- Haehnel W. Electron transport between plastoquinone and chlorophyll Ai in chloroplasts. II. Reaction kinetics and the function of plastocyanin in situ. Biochim Biophys Acta. 1977 Mar 11;459(3):418–441. doi: 10.1016/0005-2728(77)90043-3. [DOI] [PubMed] [Google Scholar]
- Haehnel W. Electron transport between plastoquinone and chlorophyll a-I in chloroplasts. Biochim Biophys Acta. 1973 Jun 28;305(3):618–631. doi: 10.1016/0005-2728(73)90081-9. [DOI] [PubMed] [Google Scholar]
- Heber U., Boardman N. K., Anderson J. M. Cytochrome b-563 redox changes in intact CO-fixing spinach chloroplasts and in developing pea chloroplasts. Biochim Biophys Acta. 1976 Feb 16;423(2):275–292. doi: 10.1016/0005-2728(76)90185-7. [DOI] [PubMed] [Google Scholar]
- Hildreth W. W. Laser-induced kinetics of cytochrome oxidation and the 518 millimicrons absorption change in spinach leaves and chloroplasts. Biochim Biophys Acta. 1968 Jan 15;153(1):197–202. doi: 10.1016/0005-2728(68)90160-6. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Laser-induced reactions of P700 and cytochrome f in a blue-green alga, Plectonema boryanum. Biochim Biophys Acta. 1971 Mar 2;226(2):320–327. doi: 10.1016/0005-2728(71)90099-5. [DOI] [PubMed] [Google Scholar]
- Horton P., Cramer W. A. The accessibility of the chloroplast cytochromes f and b-559 to ferricyanide. Biochim Biophys Acta. 1974 Dec 19;368(3):348–360. doi: 10.1016/0005-2728(74)90180-7. [DOI] [PubMed] [Google Scholar]
- Horton P., Croze E. The relationship between the activity of chloroplast photosystem II and the midpoint oxidation-reduction potential of cytochrome b-559. Biochim Biophys Acta. 1977 Oct 12;462(1):86–101. doi: 10.1016/0005-2728(77)90191-8. [DOI] [PubMed] [Google Scholar]
- Izawa S., Kraayenhof R., Ruuge E. K., Devault D. The site of KCN inhibition in the photosynthetic electron transport pathway. Biochim Biophys Acta. 1973 Sep 26;314(3):328–339. doi: 10.1016/0005-2728(73)90117-5. [DOI] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. Spectral evidence for a new photoreactive component of the oxygen-evolving system in photosynthesis. Proc Natl Acad Sci U S A. 1969 Jul;63(3):963–969. doi: 10.1073/pnas.63.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsho T. V., Kok B. Interaction between electron transport components in chloroplasts. Biochim Biophys Acta. 1970 Dec 8;223(2):240–250. doi: 10.1016/0005-2728(70)90181-7. [DOI] [PubMed] [Google Scholar]
- Ort D. R., Izawa S. Studies on the Energy-coupling Sites of Photophosphorylation: II. Treatment of Chloroplasts with NH(2)OH Plus Ethylenediaminetetraacetate to Inhibit Water Oxidation while Maintaining Energy-coupling Efficiencies. Plant Physiol. 1973 Dec;52(6):595–600. doi: 10.1104/pp.52.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rurainski H. J., Randles J., Hoch G. E. A comparative study of photosynthetic electron transport in algal cells and spinach chloroplasts. Biochim Biophys Acta. 1970;205(2):254–262. doi: 10.1016/0005-2728(70)90255-0. [DOI] [PubMed] [Google Scholar]
- Stiehl H. H., Witt H. T. Quantitative treatment of the function of plastoquinone in phostosynthesis. Z Naturforsch B. 1969 Dec;24(12):1588–1598. doi: 10.1515/znb-1969-1219. [DOI] [PubMed] [Google Scholar]
- Voegell K. K., O'keeffe D., Whitmarsh J., Dilley R. A. Valinomycin inhibition of chloroplast electron transport at or near plastoquinone. Arch Biochem Biophys. 1977 Sep;183(1):333–339. doi: 10.1016/0003-9861(77)90447-7. [DOI] [PubMed] [Google Scholar]
- WITT H. T., MUELLER A., RUMBERG B. Experimental evidence for the mechanism of photosynthesis. Nature. 1961 Jul 8;191:194–195. doi: 10.1038/191194a0. [DOI] [PubMed] [Google Scholar]
- White C. C., Chain R. K., Malkin R. Duroquinol as an electron donor for chloroplast electron transfer reactions. Biochim Biophys Acta. 1978 Apr 11;502(1):127–137. doi: 10.1016/0005-2728(78)90137-8. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bendall D. S. The reduction of plastocyanin by plastoquinol-1 in the presence of chloroplasts. A dark electron transfer reaction involving components between the two photosystems. Eur J Biochem. 1976 Jan 15;61(2):337–344. doi: 10.1111/j.1432-1033.1976.tb10027.x. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Rate of electron transfer between plastocyanin, cytochrome f, related proteins and artificial redox reagents in solution. Biochim Biophys Acta. 1974 Sep 20;357(3):370–379. doi: 10.1016/0005-2728(74)90027-9. [DOI] [PubMed] [Google Scholar]


