Abstract
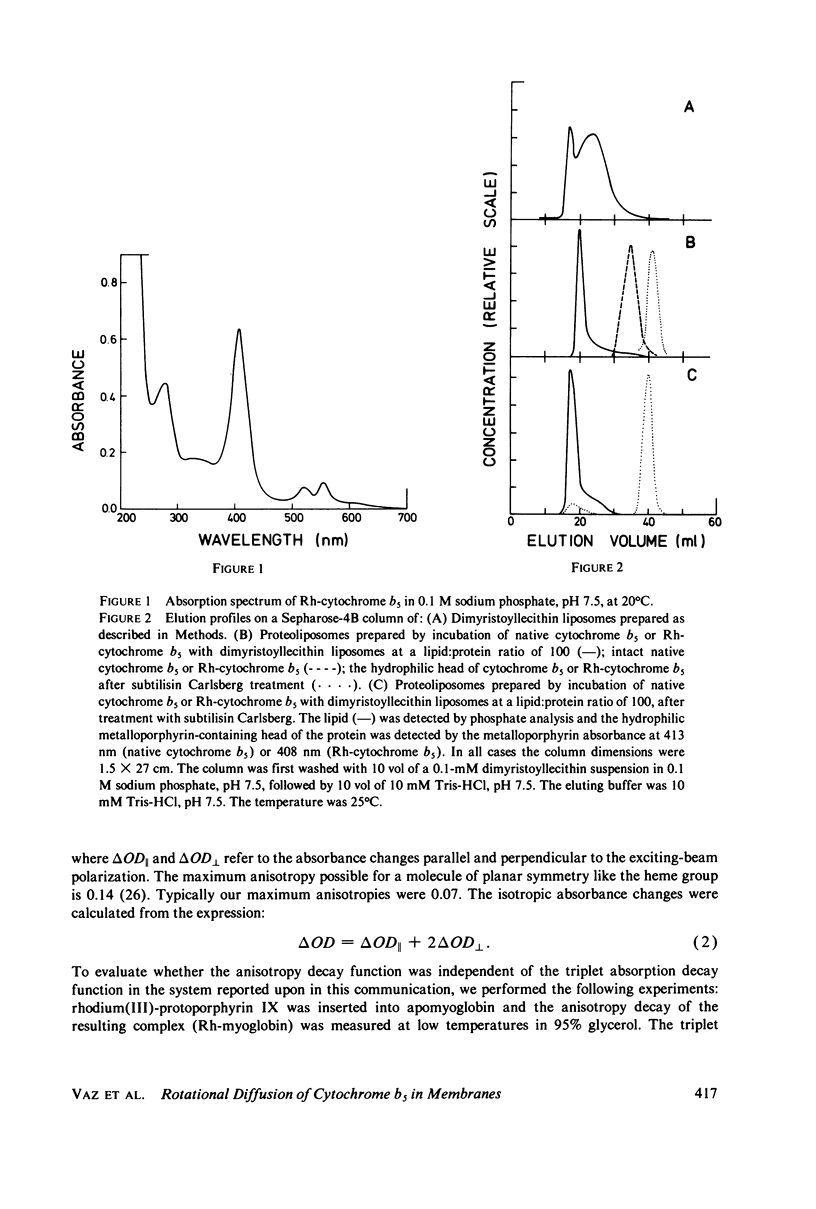
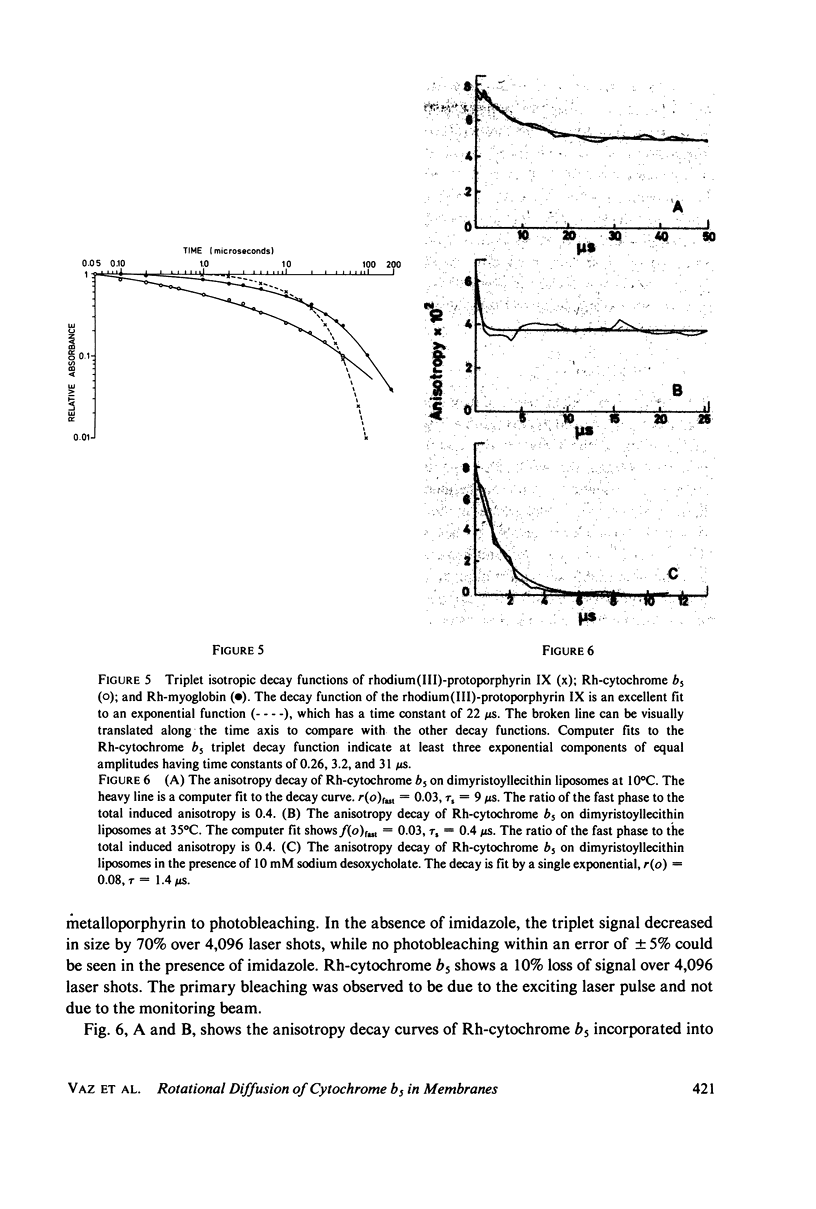
A derivative of the integral membranes protein, cytochrome b5, has been prepared in which the native heme group has been replaced by the structurally similar rhodium(III)-protoporphyrin IX. This metalloporphyrin has a finite triplet yield with a single exponential decay time of 22 microsecond in water. After insertion of the metalloporphyrin into the protein, its triplet-state decay becomes strongly nonexponential with at least three equal amplitude components with time constants varying over a range of 100. The derivatized protein has been incorporated into unilamellar liposomes prepared from dimyristoyllecithin, and the rotational diffusion of the protein in the lipid bilayer has been studied at temperatures above and below the lipid phase transition temperature via triplet absorbance anisotropy decay. The anisotropy decay curves are biphasic both above and below the lipid phase transition. The rotational diffusion constant is found to be 2.4 X 10(5) s-1 at 35 degrees C, and 1.1 X 10(4) s-1 at 10 degrees C, both being calculated from the fast decay component. The ratio of the limiting anisotropy to the initial anisotropy is 0.6 at both temperatures. This implies a cone of restricted motion of 34 degrees for the protein in the bilayer.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Chan S. S. The rate of entry of dioxygen and carbon monoxide into myoglobin. Biophys J. 1978 Oct;24(1):175–186. doi: 10.1016/S0006-3495(78)85354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. K. Rhodopsin rotates in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):35–38. doi: 10.1038/newbio236035a0. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Bürkli A., Busslinger M., Schneider G., Parish G. R. Rotational diffusion of band 3 proteins in the human erythrocyte membrane. Nature. 1976 Sep 30;263(5576):389–393. doi: 10.1038/263389a0. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Müller U., Schneider G. Rotational diffusion of bacteriorhodopsin in lipid membranes. FEBS Lett. 1977 Aug 15;80(2):465–469. doi: 10.1016/0014-5793(77)80498-5. [DOI] [PubMed] [Google Scholar]
- Cone R. A. Rotational diffusion of rhodopsin in the visual receptor membrane. Nat New Biol. 1972 Mar 15;236(63):39–43. doi: 10.1038/newbio236039a0. [DOI] [PubMed] [Google Scholar]
- Edidin M., Fambrough D. Fluidity of the surface of cultured muscle fibers. Rapid lateral diffusion of marked surface antigens. J Cell Biol. 1973 Apr;57(1):27–37. doi: 10.1083/jcb.57.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- FANELLI A. R., ANTONINI E., CAPUTO A. Studies on the structure of hemoglobin. I. Physicochemical properties of human globin. Biochim Biophys Acta. 1958 Dec;30(3):608–615. doi: 10.1016/0006-3002(58)90108-2. [DOI] [PubMed] [Google Scholar]
- Hanson L. K., Gouterman M., Hanson J. C. Porphyrins. XXIX. The crystal and molecular structure and luminescence of bis(dimethylamine)etio(I)porphinatorhodium(3) chloride dihydrate. J Am Chem Soc. 1973 Jul 25;95(15):4822–4829. doi: 10.1021/ja00796a010. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Derzko Z., Wu E. S., Hou Y., Poste G. Measurement of the lateral mobility of cell surface components in single, living cells by fluorescence recovery after photobleaching. J Supramol Struct. 1976;5(4):565(417)–576(428). doi: 10.1002/jss.400050411. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Junge W. Brownian rotation of the cytochrome oxidase in the mitochondrial inner membrane. FEBS Lett. 1972 Sep 1;25(1):109–112. doi: 10.1016/0014-5793(72)80465-4. [DOI] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Lateral diffusion of visual pigment in photorecptor disk membranes. Science. 1974 Aug 2;185(4149):457–459. doi: 10.1126/science.185.4149.457. [DOI] [PubMed] [Google Scholar]
- Mathews F. S., Levine M., Argos P. Three-dimensional Fourier synthesis of calf liver cytochrome b 5 at 2-8 A resolution. J Mol Biol. 1972 Mar 14;64(2):449–464. doi: 10.1016/0022-2836(72)90510-4. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Rogers M. J., Strittmatter P. The interaction of NADH-cytochrome b5 reductase and cytochrome b5 bound to egg lecithin liposomes. J Biol Chem. 1975 Jul 25;250(14):5713–5718. [PubMed] [Google Scholar]
- STRITTMATTER P. The nature of the heme binding in microsomal cytochrome b5. J Biol Chem. 1960 Aug;235:2492–2497. [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz W. L., Vogel H., Jähnig F., Austin R. H., Schoellmann G. Kinetics of the incorporation of cytochrome b5, an integral membrane protein, into unilamellar dimyristoyllecithin liposomes. FEBS Lett. 1978 Mar 15;87(2):269–272. doi: 10.1016/0014-5793(78)80349-4. [DOI] [PubMed] [Google Scholar]
- Zagyansky Y., Edidin M. Lateral diffusion of concanavalin A receptors in the plasma membrane of mouse fibroblasts. Biochim Biophys Acta. 1976 Apr 16;433(1):209–214. doi: 10.1016/0005-2736(76)90188-7. [DOI] [PubMed] [Google Scholar]


