Abstract
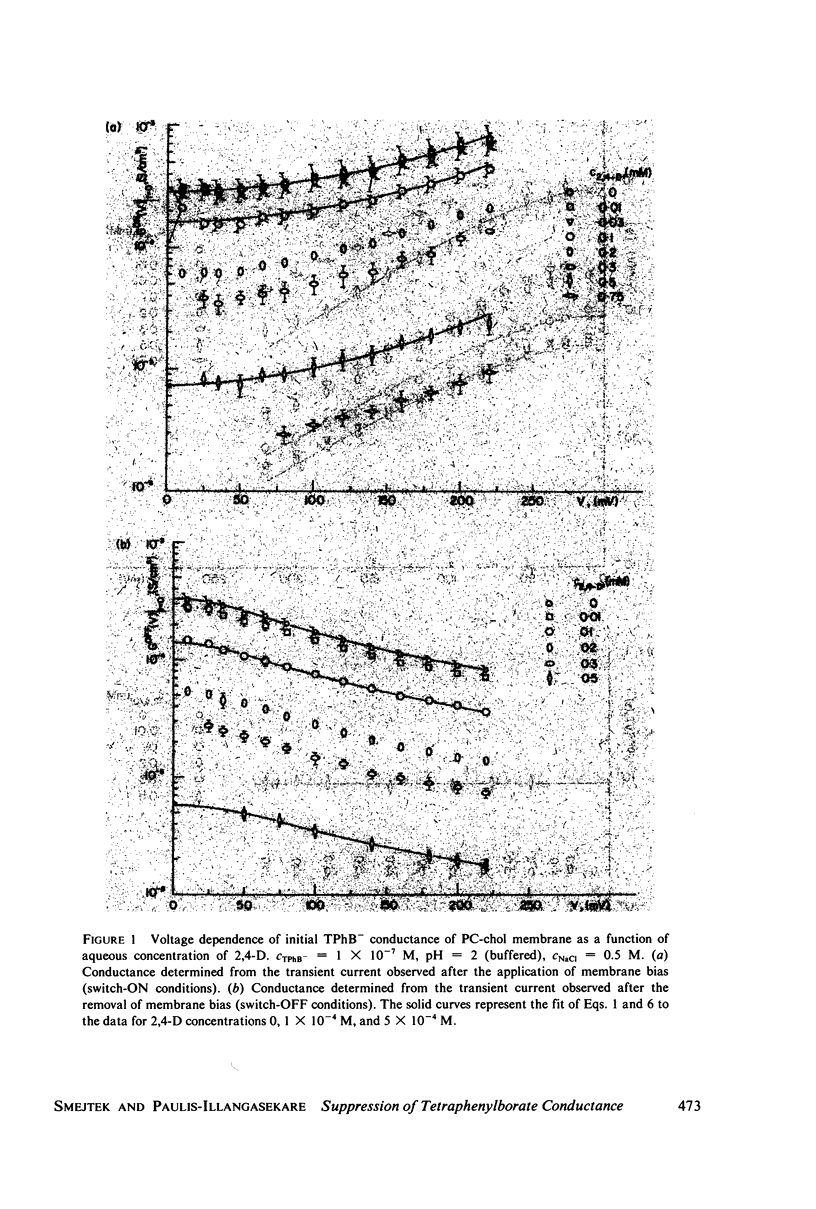
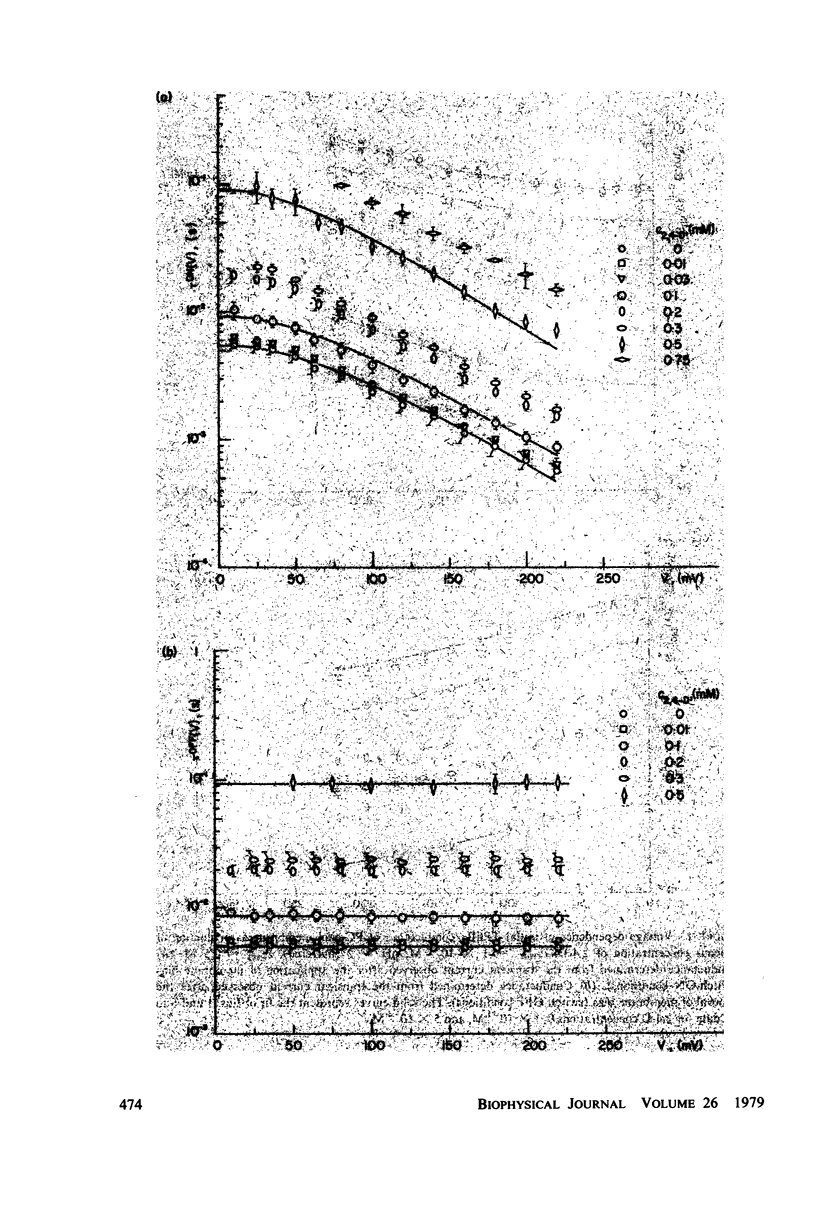
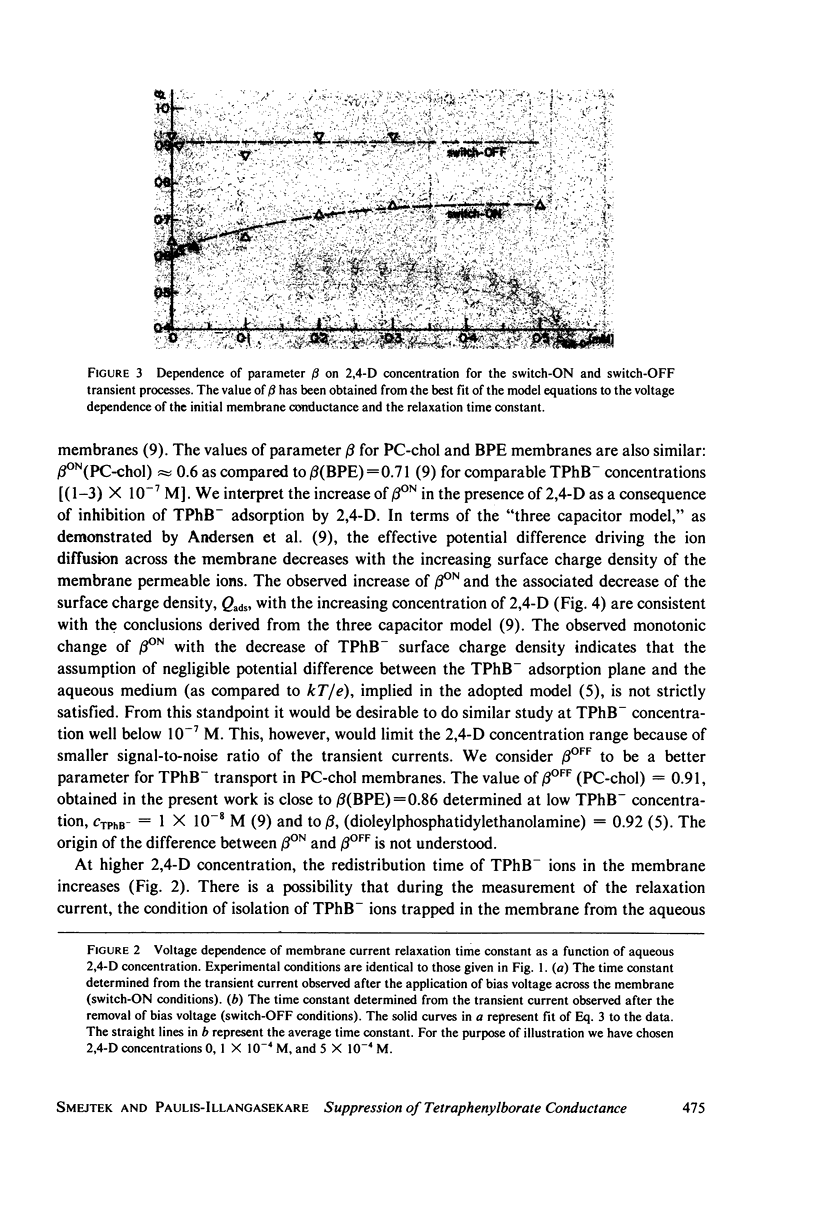
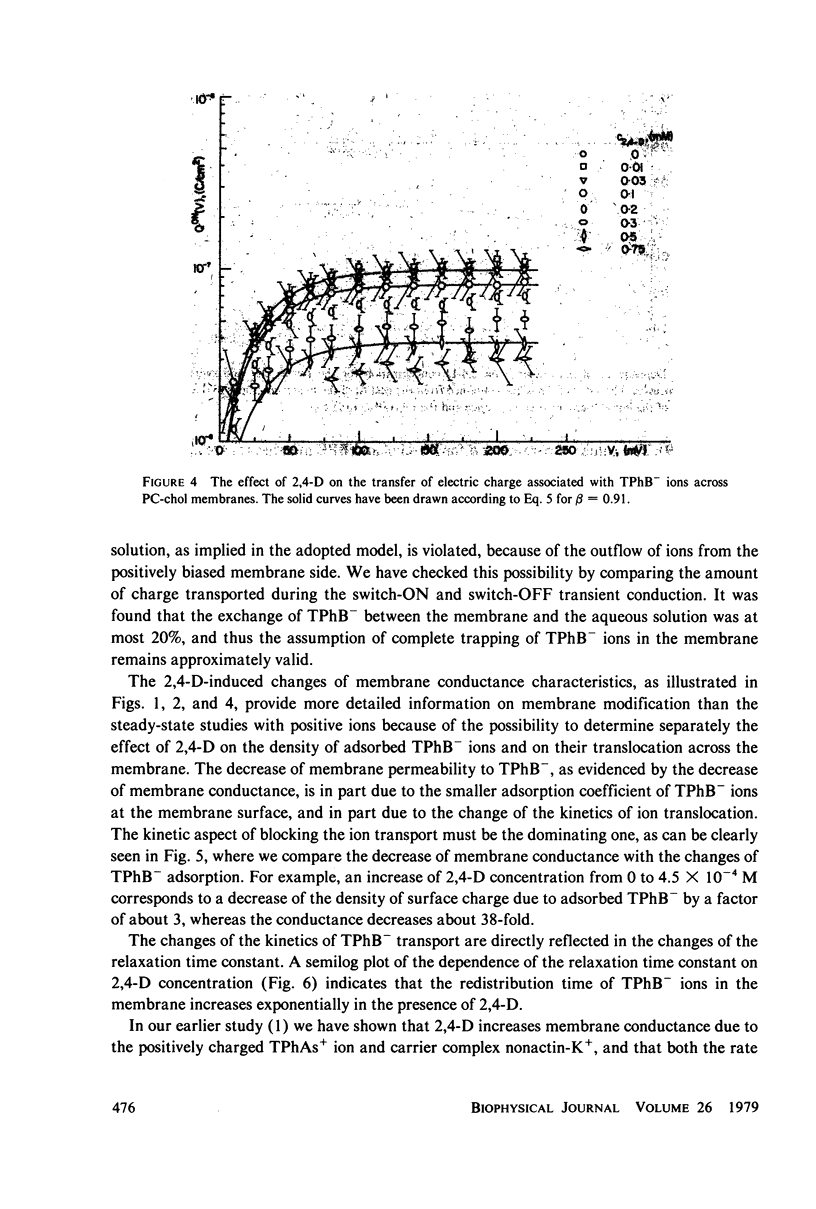
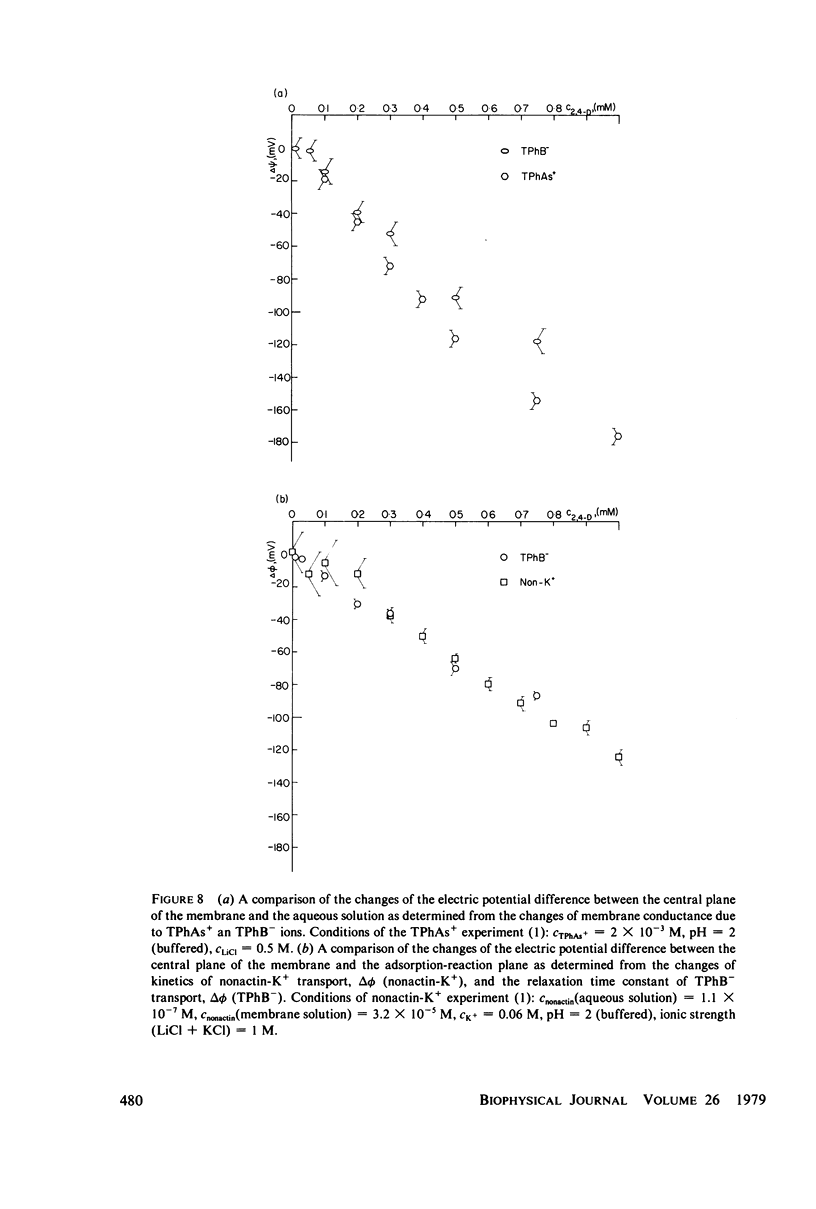
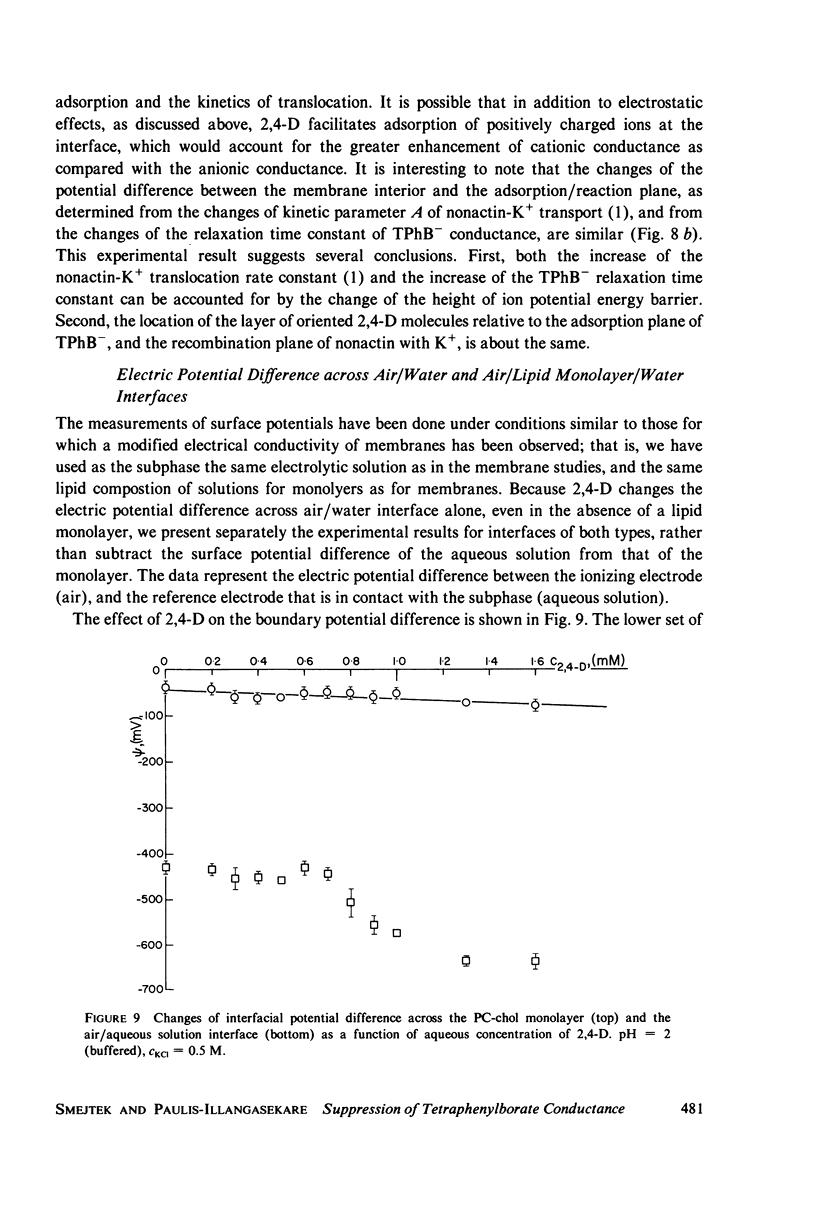
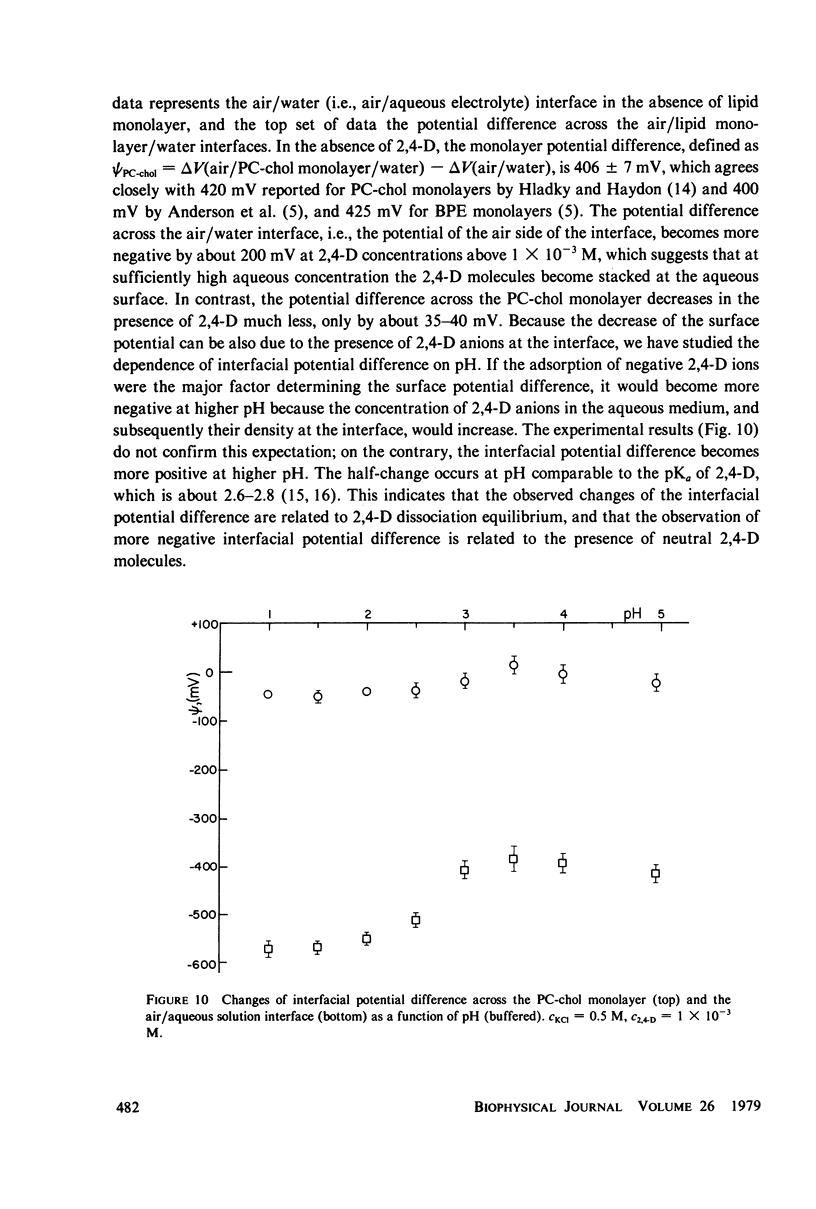
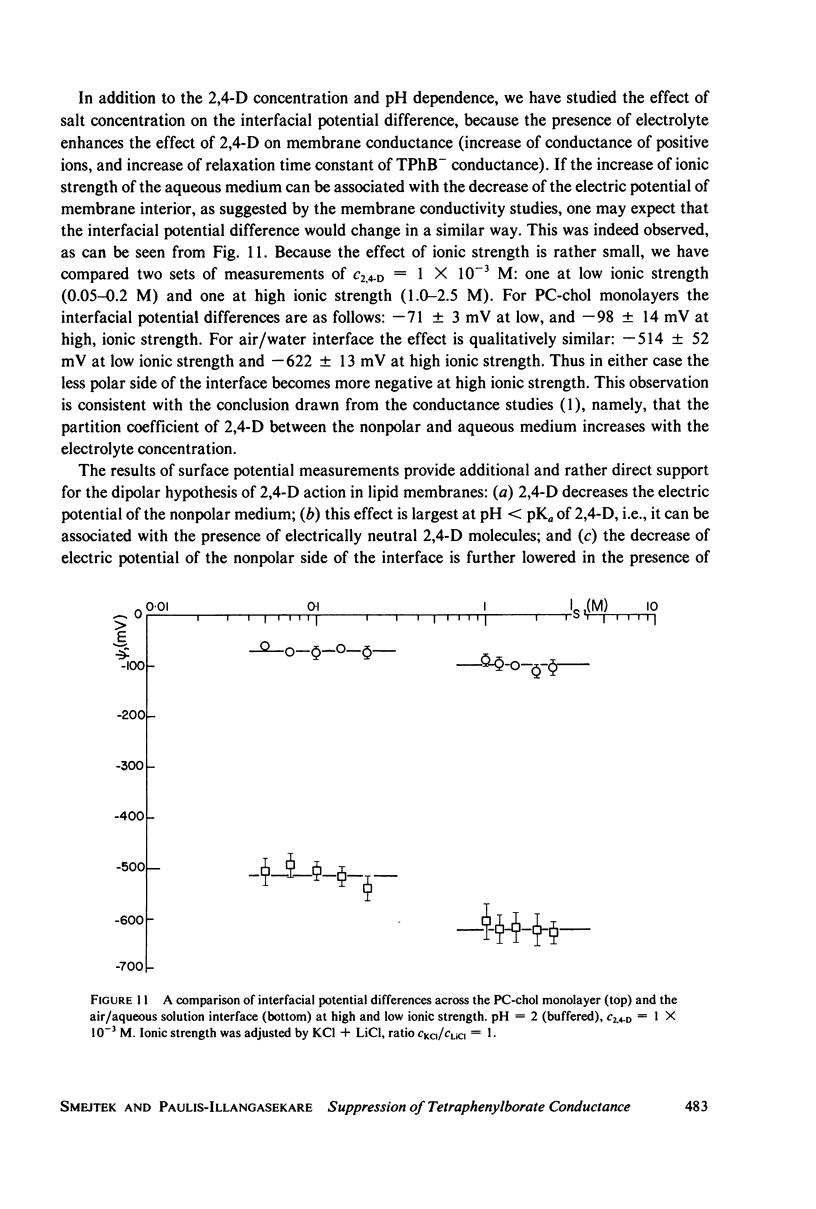
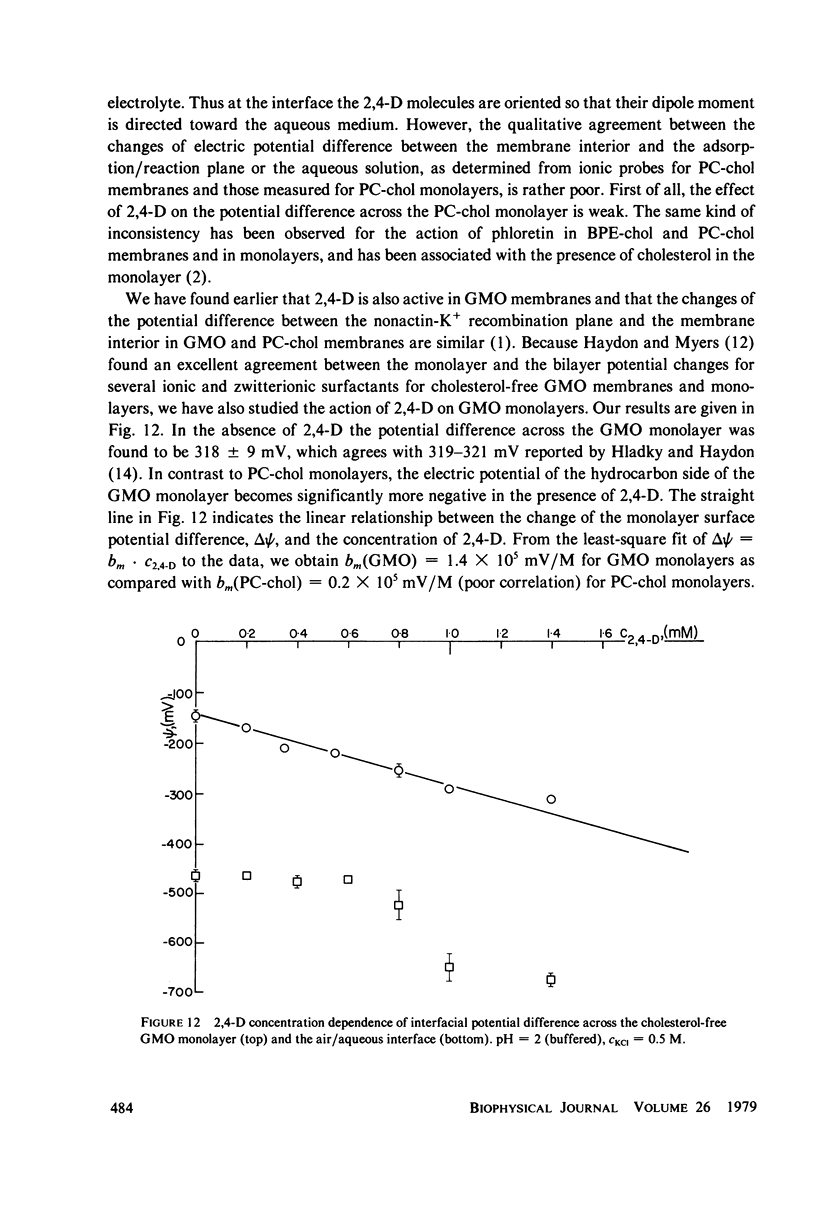
It has been shown that the blocking of negatively charged tetraphenylborate ion transport in phosphatidylcholine (PC)-cholesterol membranes by the herbicide 2,4-dichlorophenoxyacetic acid (2,4-D) is dominated by suppression of TPhB- diffusion across the membrane interior, rather than by the decrease of adsorption of TPhB- ions at the membrane surface. The blocking effect can be associated with the decrease of electric potential inside the membrane with respect to that of the aqueous medium, this decreases being proportional to the concentration of 2,4-D in the aqueous solution. It has been estimated that 25 - 30% of the total 2,4-D-induced change of the potential difference is between the plane of absorption of TPhB- and the aqueous solution, and the remaining fraction is between the membrane interior and the absorption plane. The results of this study support the dipolar hypothesis of 2,4-D action in lipid membranes. These conclusions are further supported by measurements changes of electric potential difference across air/water and air/lipid monolayer/water interfaces. It has been found that the electric potential of the nonpolar side of the interface decreases in the presence of neutral molecules of 2,4-D and that this effect becomes more prominent in presence of electrolyte. We have confirmed that PC-cholesterol monolayer cannot be considered as a model for half of the bilayer membrane because of the disagreement between the changes of the interfacial potential difference of PC-cholesterol monolayers and those determined from studied of transport of positive and negative ions across bilayer membranes. In contract, we have found close agreement between the 2,4-D-induced changes of electric potential of the lipid hydrocarbon region in glycerolmonooleate (GMO) membranes and GMO monolayers. We suggest that the action of 2,4-D in lipid membranes is not associated with the changes of orientation of dipoles of lipids constituting the membranes, but rather with a layer of 2,4-D molecules absorbed at the nonpolar/polar membrane boundary.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen O. S., Feldberg S., Nakadomari H., Levy S., McLaughlin S. Electrostatic interactions among hydrophobic ions in lipid bilayer membranes. Biophys J. 1978 Jan;21(1):35–70. doi: 10.1016/S0006-3495(78)85507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Finkelstein A., Katz I., Cass A. Effect of phloretin on the permeability of thin lipid membranes. J Gen Physiol. 1976 Jun;67(6):749–771. doi: 10.1085/jgp.67.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen O. S., Fuchs M. Potential energy barriers to ion transport within lipid bilayers. Studies with tetraphenylborate. Biophys J. 1975 Aug;15(8):795–830. doi: 10.1016/S0006-3495(75)85856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner L. J. The interaction of hydrophobic ions with lipid bilayer membranes. J Membr Biol. 1975;22(2):125–141. doi: 10.1007/BF01868167. [DOI] [PubMed] [Google Scholar]
- Gavach C., Sandeaux R. Non-mediated zero voltage conductance of hydrophobic ions through bilayer lipid membranes. Biochim Biophys Acta. 1975 Nov 17;413(1):33–44. doi: 10.1016/0005-2736(75)90056-5. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Myers V. B. Surface charge, surface dipoles and membrane conductance. Biochim Biophys Acta. 1973 May 25;307(3):429–443. doi: 10.1016/0005-2736(73)90289-7. [DOI] [PubMed] [Google Scholar]
- Melnik E., Latorre R., Hall J. E., Tosteson D. C. Phloretin-induced changes in ion transport across lipid bilayer membranes. J Gen Physiol. 1977 Feb;69(2):243–257. doi: 10.1085/jgp.69.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent D. F. An apparatus for the measurement of very small membrane relaxation currents. Anal Biochem. 1976 Jan;70(1):100–109. doi: 10.1016/s0003-2697(76)80052-8. [DOI] [PubMed] [Google Scholar]
- Smejtek P., Paulis-Illangasekare M. Modification of ion transport in lipid bilayer membranes in the presence of 2,4-dichlorophenoxyacetic acid. I. Enhancement of cationic conductance and changes of the kinetics of nonactin-mediated transport of potassium. Biophys J. 1979 Jun;26(3):441–466. doi: 10.1016/S0006-3495(79)85264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


