Abstract
A simple transporting epithelium is represented as a cellular compartment, compliant in all dimensions, and a paracellular channel, of arbitrary shape, between well-stirred mucosal ans serosal baths. The equations for mass balance, Poiseuille flow, and the Nernst-Planck equation are used to describe the continuous behavior of the system along cell and channel, whereas passive transport across membranes is given by the relations of Kedem and Katchalsky. Time-dependent terms are retained to permit study of transient phenomena. Boundary conditions at the baths demand only mass conservation and specify no a priori estimates of the system variables. A numerical model containing Na+,K+,Cl-, and impermeant cellular anions is formulated with membrane parameters taken from the literature on Necturus gallbladder. The differential equations are represented as a finite difference scheme and solved using Newton's method. It appears that apical cellular NaCl cotransport is necessary to obtain a reasonable cell chloride concentration. Investigation of the osmolality of the transepithelial flow shows that at steady state a leaky epithelium cannot separate baths of substantially different tonicity, although this does not guarantee isotonic transport between equiosmolar media. Changes in bath pressure, application of transepithelial electrical potential, and simulation of ion-substitution experiments are performed to understand the role of membrane permeabilities in determining the dynamic behavior of the epithelium.
Full text
PDF









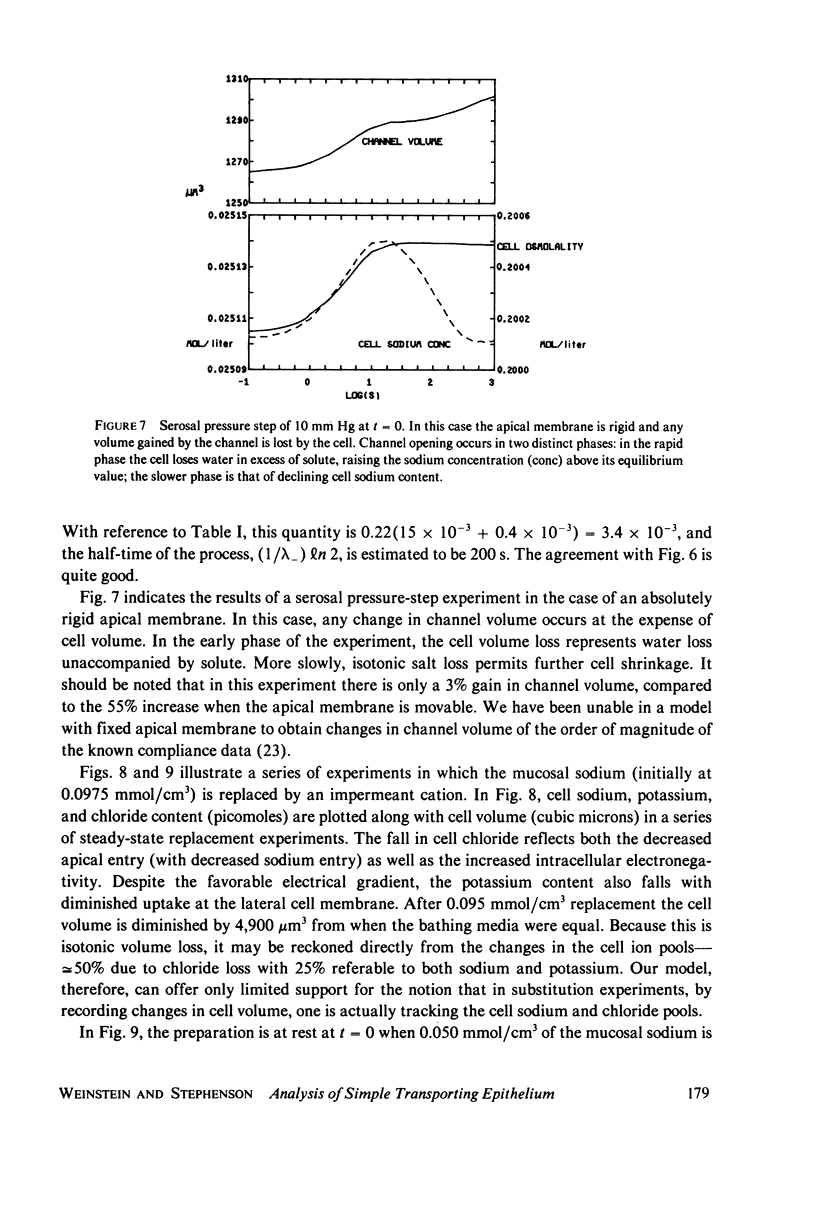
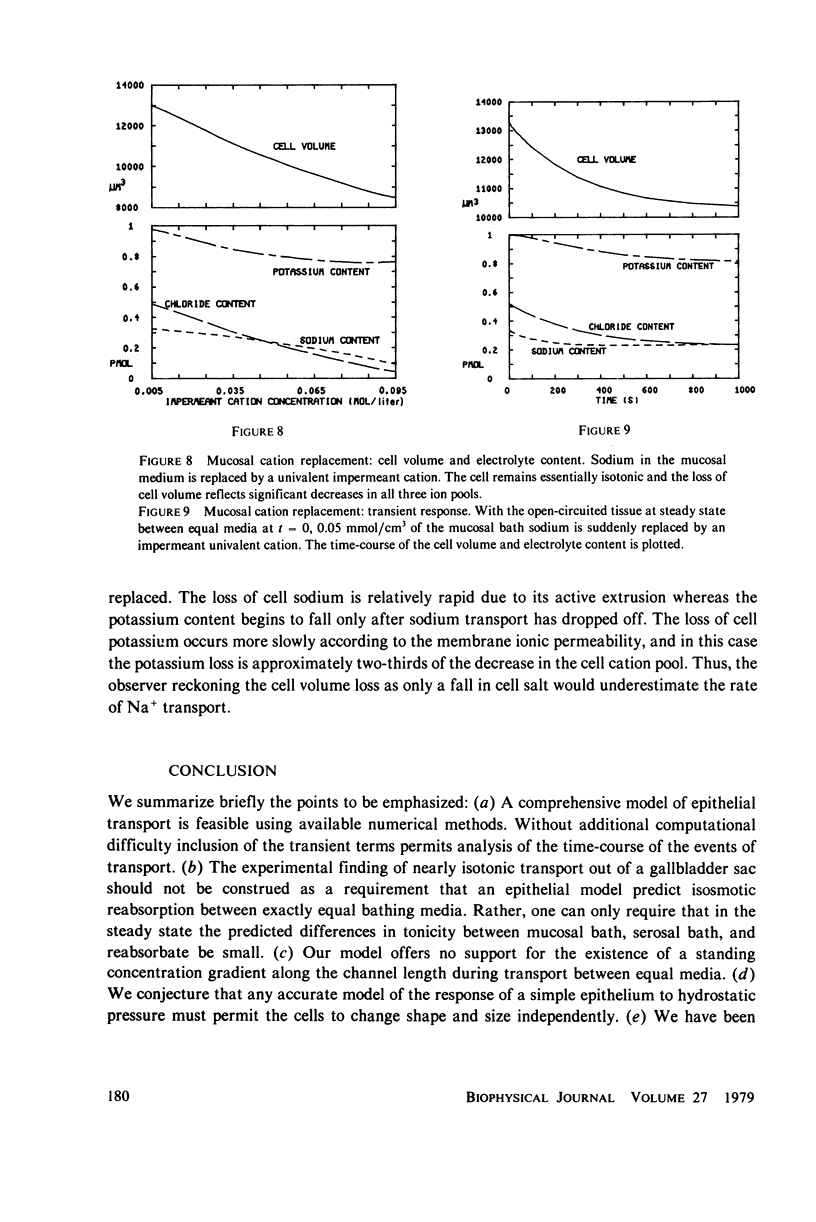

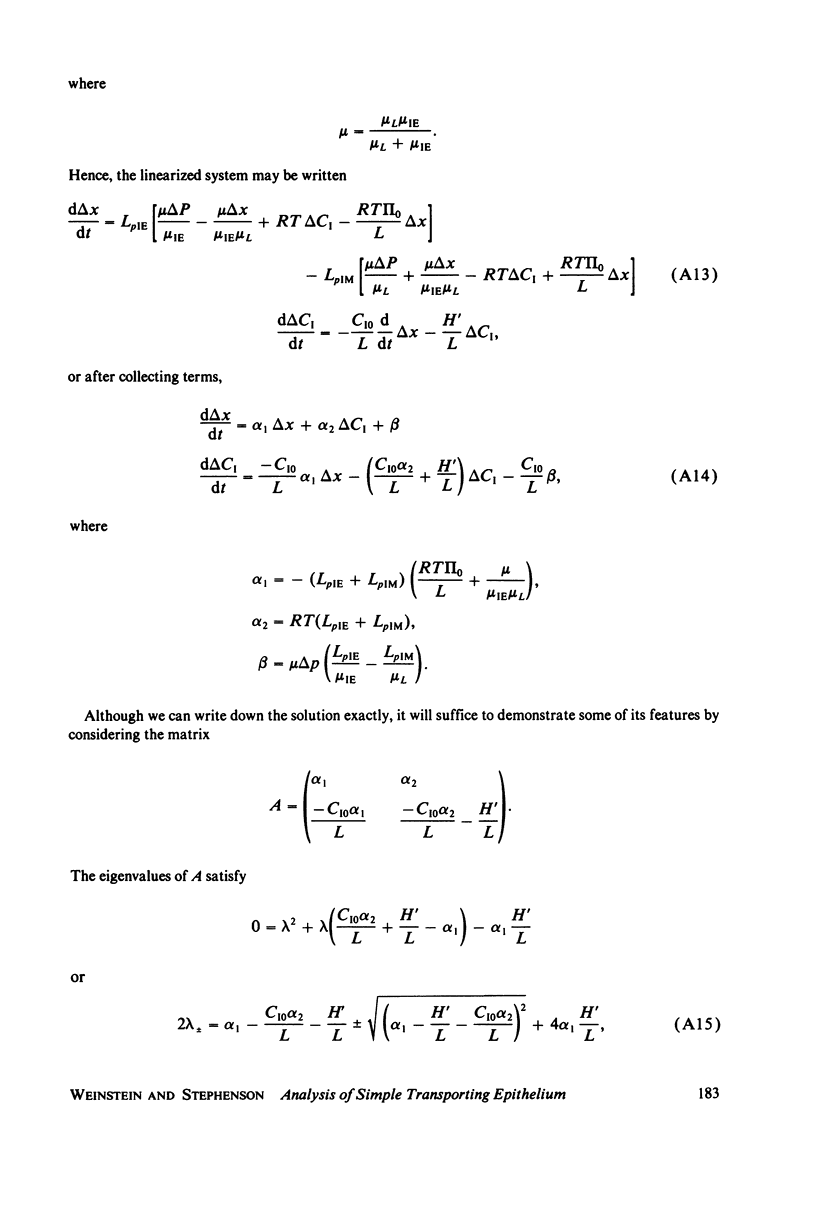



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Schafer J. A. Volume absorption in the pars recta. III. Luminal hypotonicity as a driving force for isotonic volume absorption. Am J Physiol. 1978 Apr;234(4):F349–F355. doi: 10.1152/ajprenal.1978.234.4.F349. [DOI] [PubMed] [Google Scholar]
- Bindslev N., Tormey J. M., Wright E. M. The effects of electrical and osmotic gradients on lateral intercellular spaces and membrane conductance in a low resistance epithelium. J Membr Biol. 1974;19(4):357–380. doi: 10.1007/BF01869986. [DOI] [PubMed] [Google Scholar]
- Blom H., Helander H. F. Quantitative electron microscopical studies on in vitro incubated rabbit gallbladder epithelium. J Membr Biol. 1977 Oct 3;37(1):45–61. doi: 10.1007/BF01940923. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. The mechanism of solute transport by the gall-bladder. J Physiol. 1962 May;161:474–502. doi: 10.1113/jphysiol.1962.sp006899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M., Bossert W. H. Standing-gradient osmotic flow. A mechanism for coupling of water and solute transport in epithelia. J Gen Physiol. 1967 Sep;50(8):2061–2083. doi: 10.1085/jgp.50.8.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E. The route of passive ion movement through the epithelium of Necturus gallbladder. J Membr Biol. 1972;8(3):259–301. doi: 10.1007/BF01868106. [DOI] [PubMed] [Google Scholar]
- Hill A. E. Solute-solvent coupling in epithelia: a critical examination of the standing-gradient osmotic flow theory. Proc R Soc Lond B Biol Sci. 1975 Jun 20;190(1098):99–114. doi: 10.1098/rspb.1975.0081. [DOI] [PubMed] [Google Scholar]
- Huss R. E., Marsh D. J. A model of NaCl and water flow through paracellular pathways of renal proximal tubules. J Membr Biol. 1975;23(3-4):305–347. doi: 10.1007/BF01870256. [DOI] [PubMed] [Google Scholar]
- Olivares W., McQuarrie D. A. On the theory of ionic solutions. Biophys J. 1975 Feb;15(2 Pt 1):143–162. doi: 10.1016/s0006-3495(75)85798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. II. Ionic permeability of the apical cell membrane. J Membr Biol. 1975 Dec 4;25(1-2):141–161. doi: 10.1007/BF01868572. [DOI] [PubMed] [Google Scholar]
- Sackin H., Boulpaep E. L. Models for coupling of salt and water transport; Proximal tubular reabsorption in Necturus kidney. J Gen Physiol. 1975 Dec;66(6):671–733. doi: 10.1085/jgp.66.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer J. A., Patlak C. S., Andreoli T. E. A component of fluid absorption linked to passive ion flows in the superficial pars recta. J Gen Physiol. 1975 Oct;66(4):445–471. doi: 10.1085/jgp.66.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring K. R., Hope A. Size and shape of the lateral intercellular spaces in a living epithelium. Science. 1978 Apr 7;200(4337):54–58. doi: 10.1126/science.635571. [DOI] [PubMed] [Google Scholar]
- Stephenson J. L. Analysis of the transient behavior of kidney models. Bull Math Biol. 1978;40(2):211–221. doi: 10.1007/BF02461436. [DOI] [PubMed] [Google Scholar]
- Stephenson J. L., Mejia R., Tewarson R. P. Model of solute and water movement in the kidney. Proc Natl Acad Sci U S A. 1976 Jan;73(1):252–256. doi: 10.1073/pnas.73.1.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. L., Tewarson R. P., Mejia R. Quantitative analysis of mass and energy balance in non-ideal models of the renal counterflow system. Proc Natl Acad Sci U S A. 1974 May;71(5):1618–1622. doi: 10.1073/pnas.71.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling L. W., Grantham J. J. Physical properties of isolated perfused renal tubules and tubular basement membranes. J Clin Invest. 1972 May;51(5):1063–1075. doi: 10.1172/JCI106898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J. Shape of epithelial cells and intercellular channels in the rabbit proximal nephron. Kidney Int. 1976 May;9(5):385–394. doi: 10.1038/ki.1976.48. [DOI] [PubMed] [Google Scholar]
- Welling L. W., Welling D. J. Surface areas of brush border and lateral cell walls in the rabbit proximal nephron. Kidney Int. 1975 Dec;8(6):343–348. doi: 10.1038/ki.1975.125. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Slegers J. F. The electrical potential profile of gallbladder epithelium. J Membr Biol. 1975 Dec 4;24(3-4):341–363. doi: 10.1007/BF01868631. [DOI] [PubMed] [Google Scholar]


