Abstract
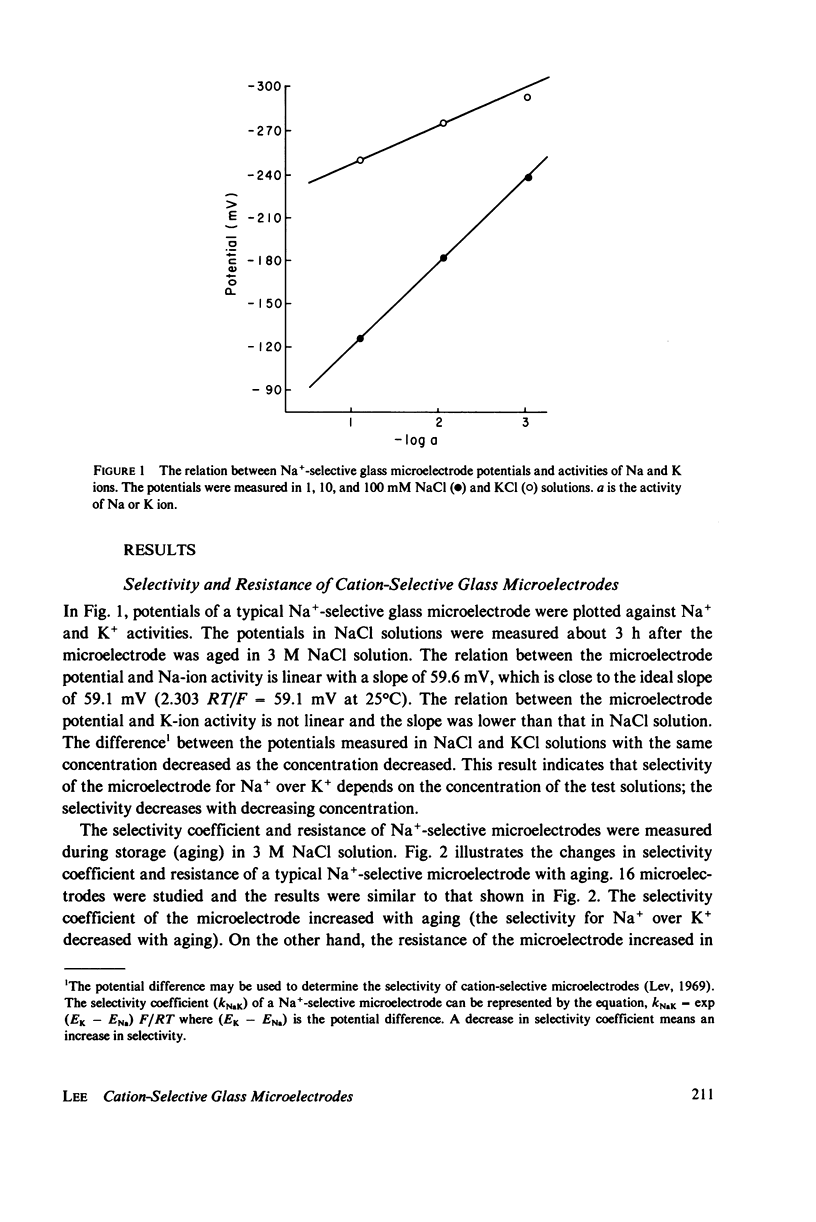
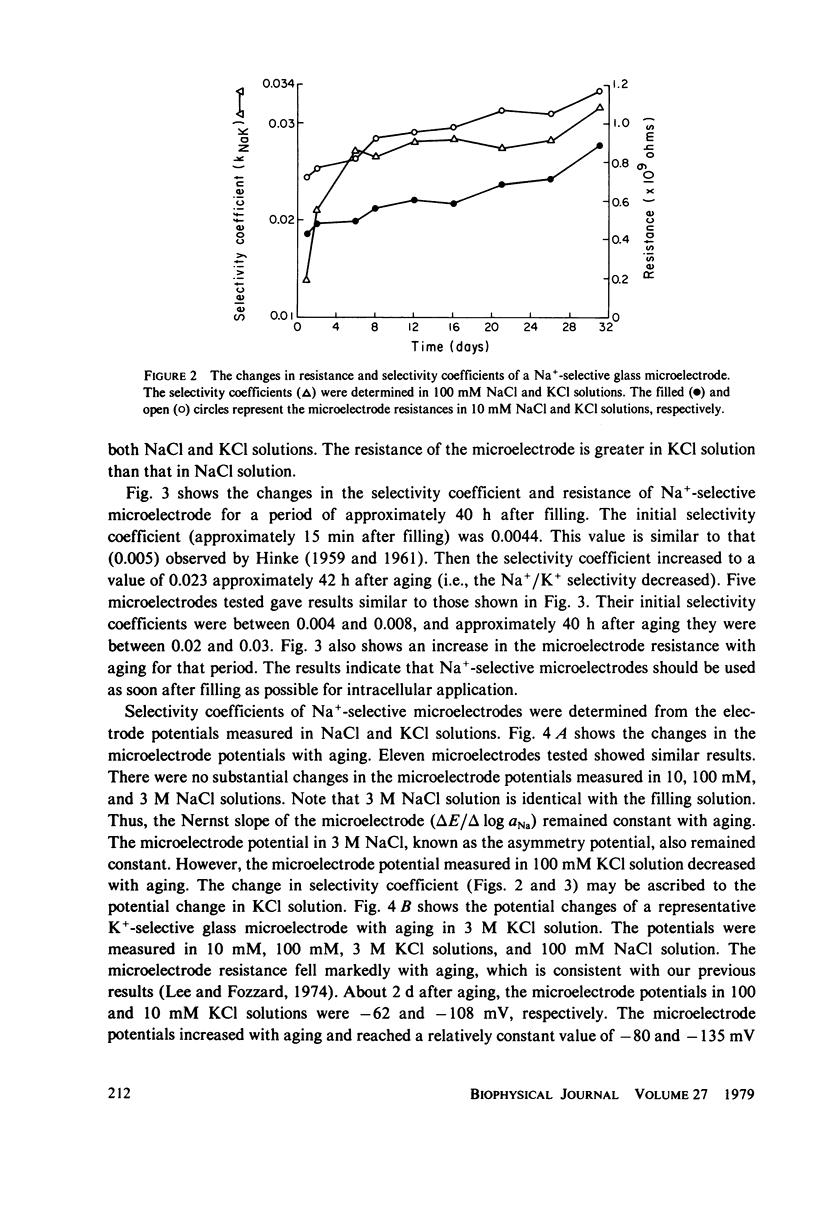
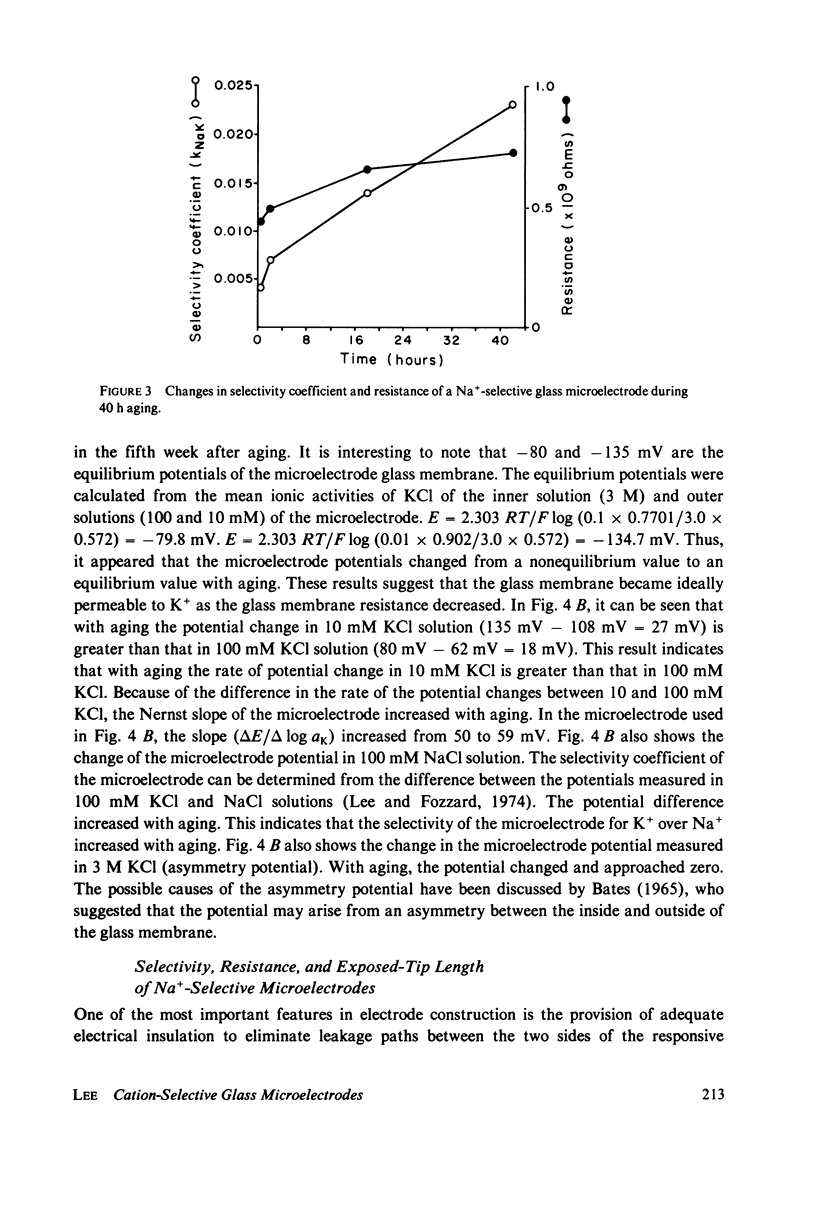
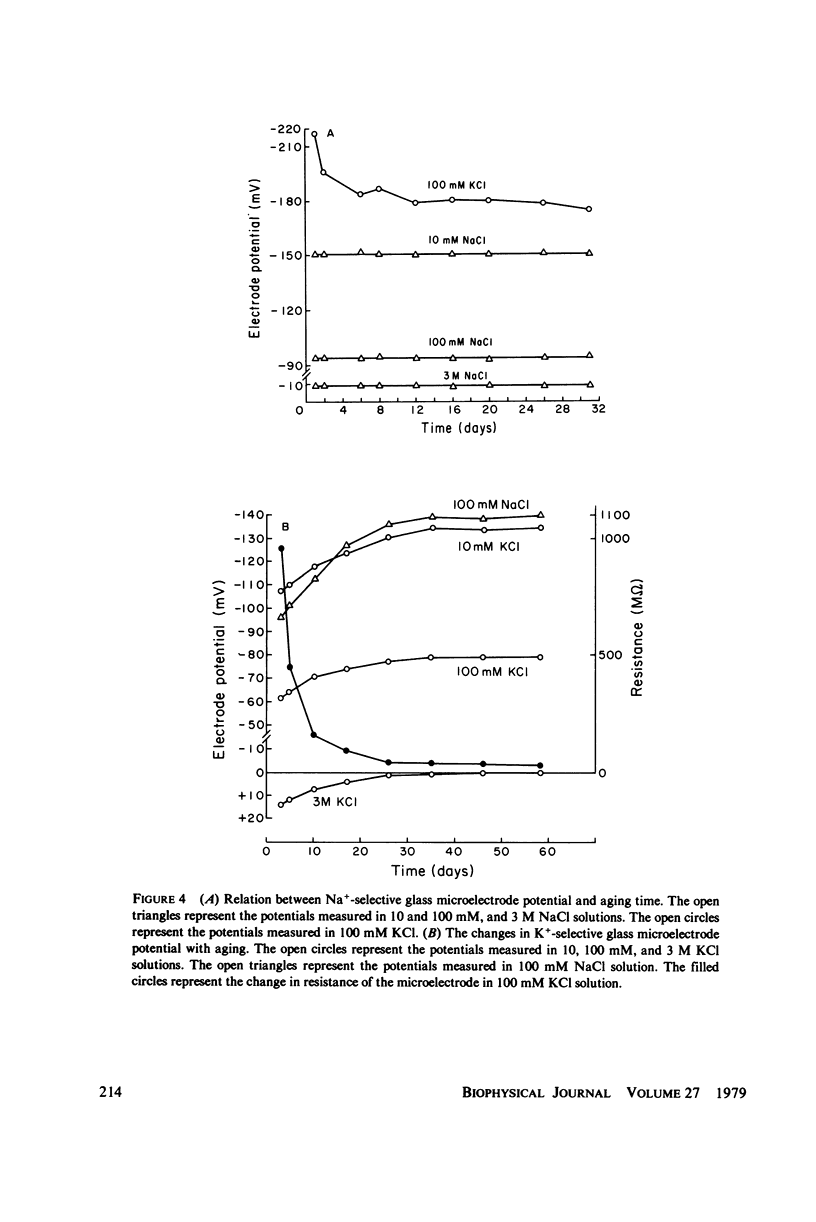
Electrochemical properties of Na+-selective glass microelectrodes were studied and compared with those of K+-selective glass microelectrodes. The selectivity of Na+-selective glass microelectrodes depended on the ion concentration of test solutions. With aging, resistance of Na+-selective microelectrodes increased and their selectivity for Na over K decreased. Na+-selective microelectrodes potential measured in NaCl solution remained constant with aging, while the potential measured in KCl solution decreased and became more positive. The changes in resistance and potential of Na+-selective microelectrodes may be due to the effects of the less mobile cation, i.e., H+ or K+ on the Na ion exchange in the Na-sensing region. The results indicate that Na+-selective microelectrodes must be used as soon after filling as possible. The selectivity of Na+-selective microelectrodes increased with increase of the sensitive exposed-tip length, whereas their response time became slow due to a large recessed volume, indicating requirement of an optimum exposed-tip length for intracellular applications. The changes in the properties of Na+-selective glass microelectrodes with aging contrasted with those of K+-selective glass microelectrodes in which resistance decreased and K+-selectivity increased. The K+-selective microelectrodes required aging before use for a high selectivity and low resistance. The K+-selective microelectrodes with low resistance after sufficient aging can be used without insulation to measure K+ and Na+ activities in aqueous solutions. The different properties between Na+- and K+-selective microelectrodes are understandable, because hydration of N+-selective glass is much less extensive than that of K+-selective glass.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENMAN G., RUDIN D. O., CASBY J. U. Glass electrode for measuring sodium ion. Science. 1957 Oct 25;126(3278):831–834. doi: 10.1126/science.126.3278.831. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Sandblom J. P., Walker J. L., Jr Membrane structure and ion permeation. Study of ion exchange membrane structure and function is relevant to analysis of biological ion permeation. Science. 1967 Feb 24;155(3765):965–974. doi: 10.1126/science.155.3765.965. [DOI] [PubMed] [Google Scholar]
- HINKE J. A. Glass micro-electrodes for measuring intracellular activities of sodium and potassium. Nature. 1959 Oct 17;184(Suppl 16):1257–1258. doi: 10.1038/1841257a0. [DOI] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Armstrong W. M. State and distribution of potassium and sodium ions in frog skeletal muscle. J Membr Biol. 1974;15(4):331–362. doi: 10.1007/BF01870094. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Electrochemical properties of hydrated cation-selective glass membrane. A model of K+ and Na+ transport. Biophys J. 1974 Jan;14(1):46–68. doi: 10.1016/S0006-3495(74)85902-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. L., Brown H. M. Intracellular ionic activity measurements in nerve and muscle. Physiol Rev. 1977 Oct;57(4):729–778. doi: 10.1152/physrev.1977.57.4.729. [DOI] [PubMed] [Google Scholar]


