Abstract
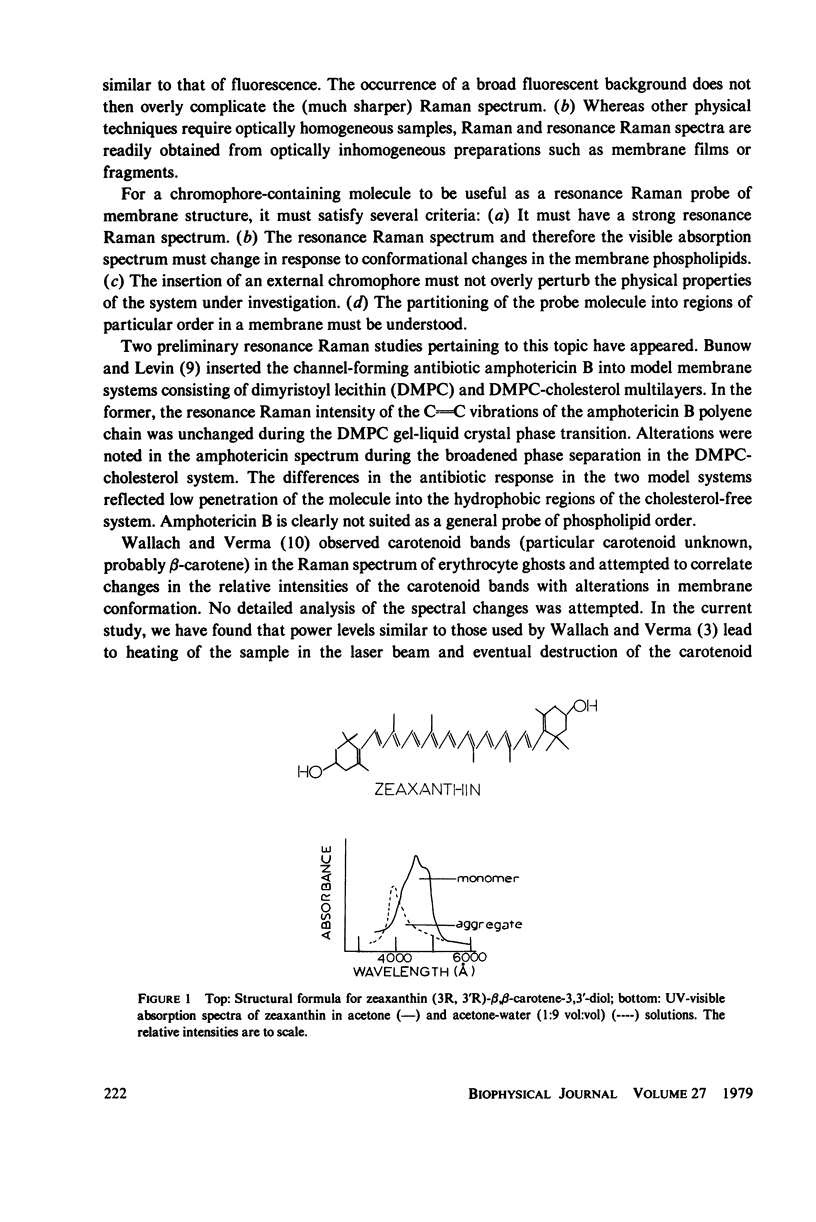
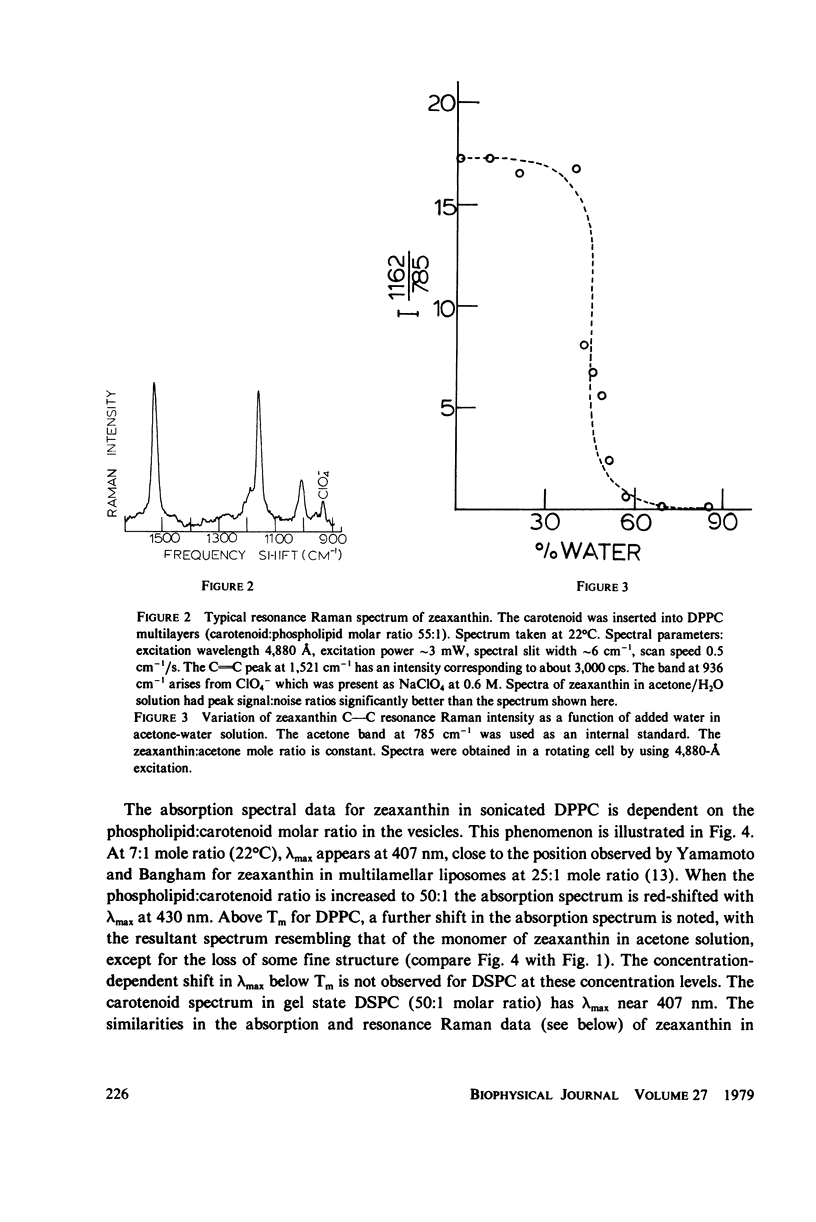
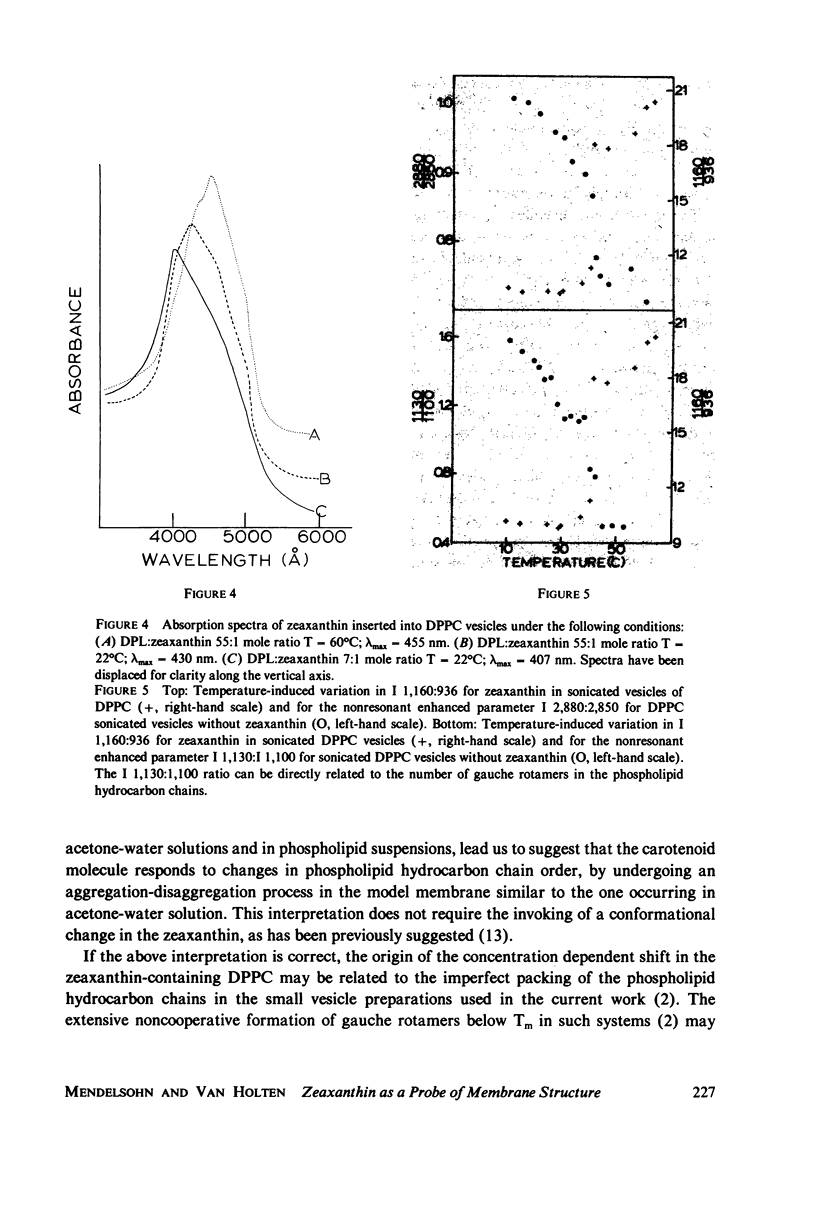
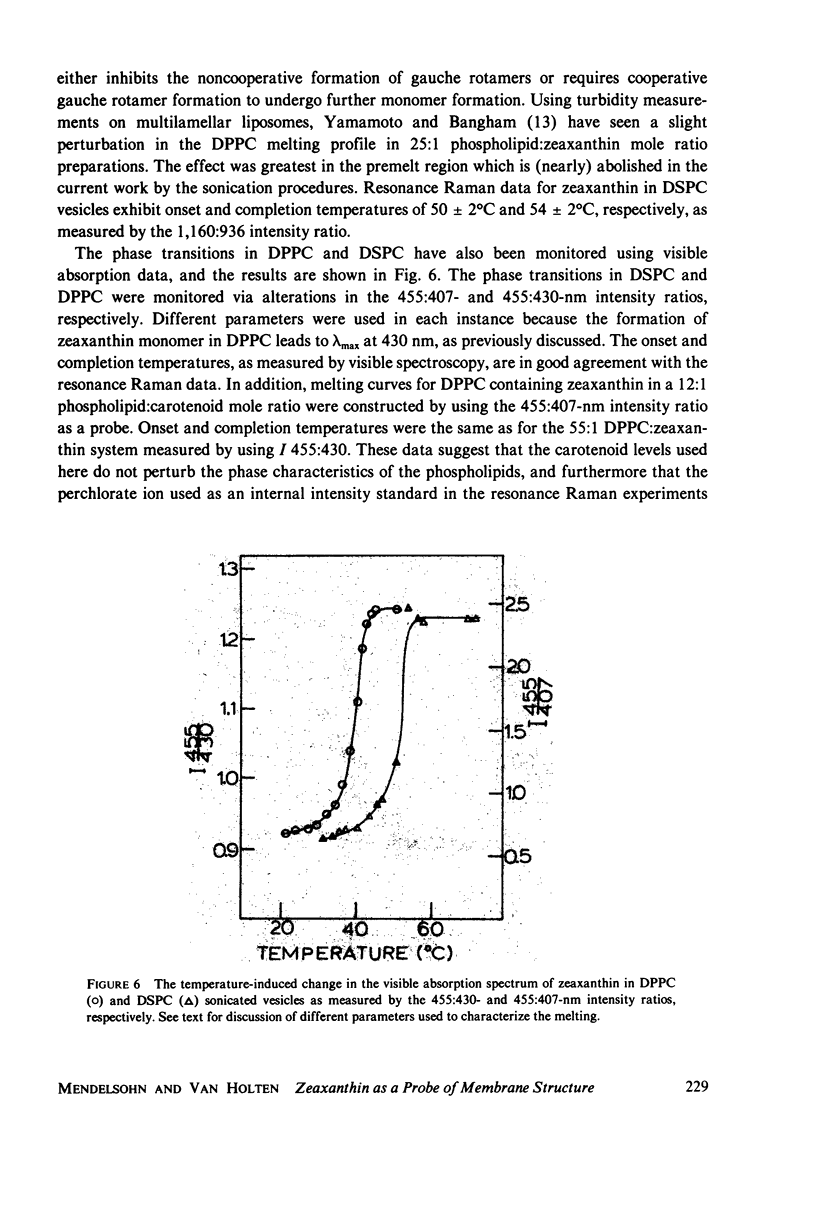
When zeaxanthin ([3R,3R']-beta, beta-carotene-3,3'diol) is inserted into phospholipid dispersions and the latter heated through their gel-liquid crystal phase transitions, large changes are noted in the resonance Raman and absorption spectra of the carotenoid molecule. By analogy with the data of Carey and co-workers (J. Raman Spectrosc. 6:282) who studied the aggregation of zeaxanthin in acetone-water solutions, it is suggested that the carotenoid aggregates in the phospholipid gel state while forming a monomer in liquid crystal phases. The alterations in both the visible absorption and resonance Raman data have been used to monitor phospholipid phase behavior in dipalmitoylphosphatidylcholine and distearoylphosphatidylcholine, (DSPC) one-component systems and binary mixtures. The phase diagram obtained for the binary system, as constructed from visible absorption and resonance Raman data, is compared with that of Shimshick and McConnell (Biochemistry. 12:2351) obtained from electron spin resonance (ESR) studies. Although the agreement between absorption and ESR data is generally satisfactory, onset temperatures for phase separation at low DSPC mole fractions deduced from resonance Raman measurements are several degrees lower than those from the other methods. Nevertheless, the use of zeaxanthin as a resonance Raman and visible absorption probe behavior will be useful in some situations where ordinary Raman spectroscopic data cannot be obtained easily. The advantage of the resonance Raman approach is illustrated in a study of the phase behavior of a phospholipid extract of a cel- mutant of Neurospora crassa. A phase separation region is observed with onset and completion temperatures of -19 and -6 degrees C, respectively.
Full text
PDF





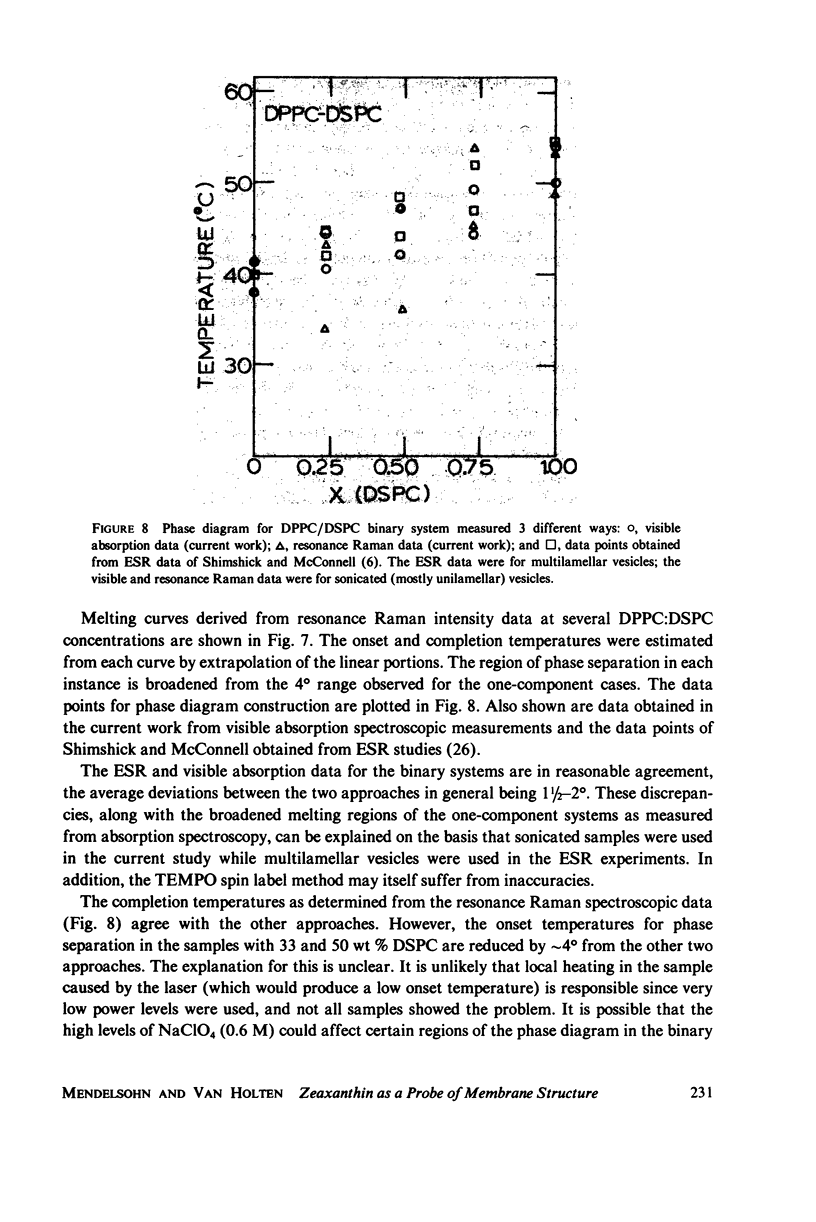

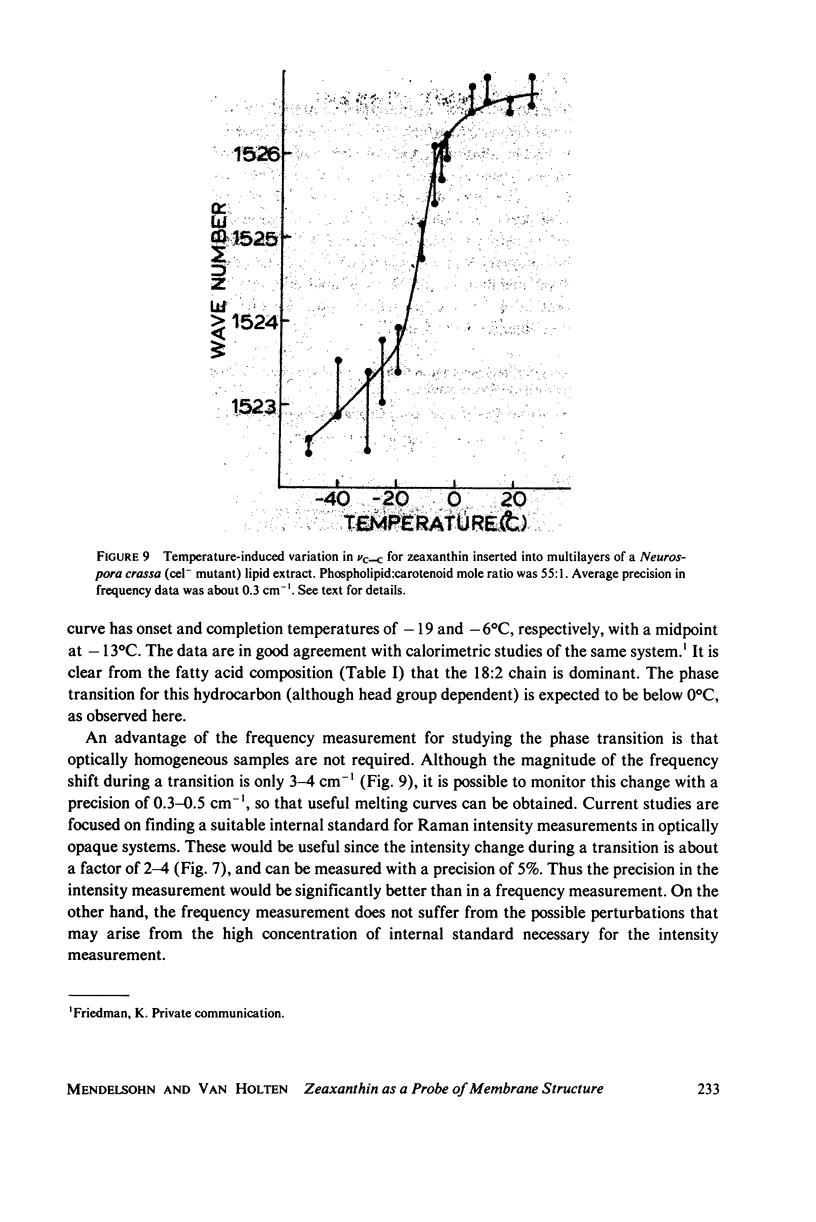


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A., Ackermann T. A calorimetric study of the lipid phase transitions in aqueous dispersions of phosphorylcholine--phosphorylethanolamine mixtures. FEBS Lett. 1974 Jul 1;43(1):71–74. doi: 10.1016/0014-5793(74)81108-7. [DOI] [PubMed] [Google Scholar]
- Bunow M. R., Levin I. W. Vibrational Raman spectra of lipid systems containing amphotericin B. Biochim Biophys Acta. 1977 Jan 4;464(1):202–216. doi: 10.1016/0005-2736(77)90382-0. [DOI] [PubMed] [Google Scholar]
- Chapman D., Urbina J. Biomembrane phase transitions. Studies of lipid-water systems using differential scanning calorimetry. J Biol Chem. 1974 Apr 25;249(8):2512–2521. [PubMed] [Google Scholar]
- Gaber B. P., Peticolas W. L. On the quantitative interpretation of biomembrane structure by Raman spectroscopy. Biochim Biophys Acta. 1977 Mar 1;465(2):260–274. doi: 10.1016/0005-2736(77)90078-5. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Lipid phase transitions and phase diagrams. II. Mictures involving lipids. Biochim Biophys Acta. 1977 Nov 14;472(3-4):285–344. doi: 10.1016/0304-4157(77)90001-6. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Gorczyca L. E., Meiklejohn G. A laser Raman spectroscopic investigation of phospholipid and protein configurations in hemoglobin-free erythrocyte ghosts. Biochim Biophys Acta. 1975 Feb 28;382(1):51–57. doi: 10.1016/0005-2736(75)90371-5. [DOI] [PubMed] [Google Scholar]
- Luna E. J., McConnell H. M. Lateral phase separations in binary mixtures of phospholipids having different charges and different crystalline structures. Biochim Biophys Acta. 1977 Oct 17;470(2):303–316. doi: 10.1016/0005-2736(77)90108-0. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Investigation of phase transitions of lipids and lipid mixtures by sensitivity differential scanning calorimetry. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3862–3866. doi: 10.1073/pnas.73.11.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn R., Sunder S., Bernstein H. J. The effect of sonication on the hydrocarbon chain conformation in model membrane systems: a Raman spectroscopic study. Biochim Biophys Acta. 1976 Feb 6;419(3):563–569. doi: 10.1016/0005-2736(76)90270-4. [DOI] [PubMed] [Google Scholar]
- Mendelsohn R., Taraschi T. Deuterated phospholipids as Raman spectroscopic probes of membrane structure: dipalmitoylphosphatidylcholine-dipalmitoylphosphatidylethanolamine multilayers. Biochemistry. 1978 Sep 19;17(19):3944–3949. doi: 10.1021/bi00612a010. [DOI] [PubMed] [Google Scholar]
- Milanovich F. P., Shore B., Harney R. C., Tu A. T. Raman spectroscopic analysis of Dutch Belt rabbit erythrocyte ghosts. Chem Phys Lipids. 1976 Sep;17(1):79–84. doi: 10.1016/0009-3084(76)90038-4. [DOI] [PubMed] [Google Scholar]
- Rimai L., Heyde M. E., Gill D. Vibrational spectra of some carotenoids and related linear polyenes. A Raman spectroscopic study. J Am Chem Soc. 1973 Jul 11;95(14):4493–4501. doi: 10.1021/ja00795a005. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Andrew J. R., De Grip W. J., Stanley H. E. Opsin structure probed by raman spectroscopy of photoreceptor membranes. Science. 1976 Mar 19;191(4232):1176–1178. doi: 10.1126/science.1257742. [DOI] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separation in phospholipid membranes. Biochemistry. 1973 Jun 5;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Petersen M., Diamond J. Conjugated polyene fatty acids on fluorescent probes: spectroscopic characterization. Biochemistry. 1977 Mar 8;16(5):813–819. doi: 10.1021/bi00624a001. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Suurkuusk J., Lentz B. R., Barenholz Y., Biltonen R. L., Thompson T. E. A calorimetric and fluorescent probe study of the gel-liquid crystalline phase transition in small, single-lamellar dipalmitoylphosphatidylcholine vesicles. Biochemistry. 1976 Apr 6;15(7):1393–1401. doi: 10.1021/bi00652a007. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F. Erythrocyte membranes undergo cooperative, pH-sensitive state transitions in the physiological temperature range: evidence from Raman spectroscopy. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3558–3561. doi: 10.1073/pnas.73.10.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Verma S. P. Raman and resonance-Raman scattering by erythrocyte ghosts. Biochim Biophys Acta. 1975 Apr 8;382(4):542–551. doi: 10.1016/0005-2736(75)90221-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Bangham A. D. Carotenoid organization in membranes. Thermal transition and spectral properties of carotenoid-containing liposomes. Biochim Biophys Acta. 1978 Feb 2;507(1):119–127. doi: 10.1016/0005-2736(78)90379-6. [DOI] [PubMed] [Google Scholar]
- Yellin N., Levin I. W. Hydrocarbon chain disorder in lipid bilayers. Temperature dependent Raman spectra of 1,2-diacyl phosphatidylcholine-water gels. Biochim Biophys Acta. 1977 Nov 24;489(2):177–190. doi: 10.1016/0005-2760(77)90137-0. [DOI] [PubMed] [Google Scholar]


