Abstract
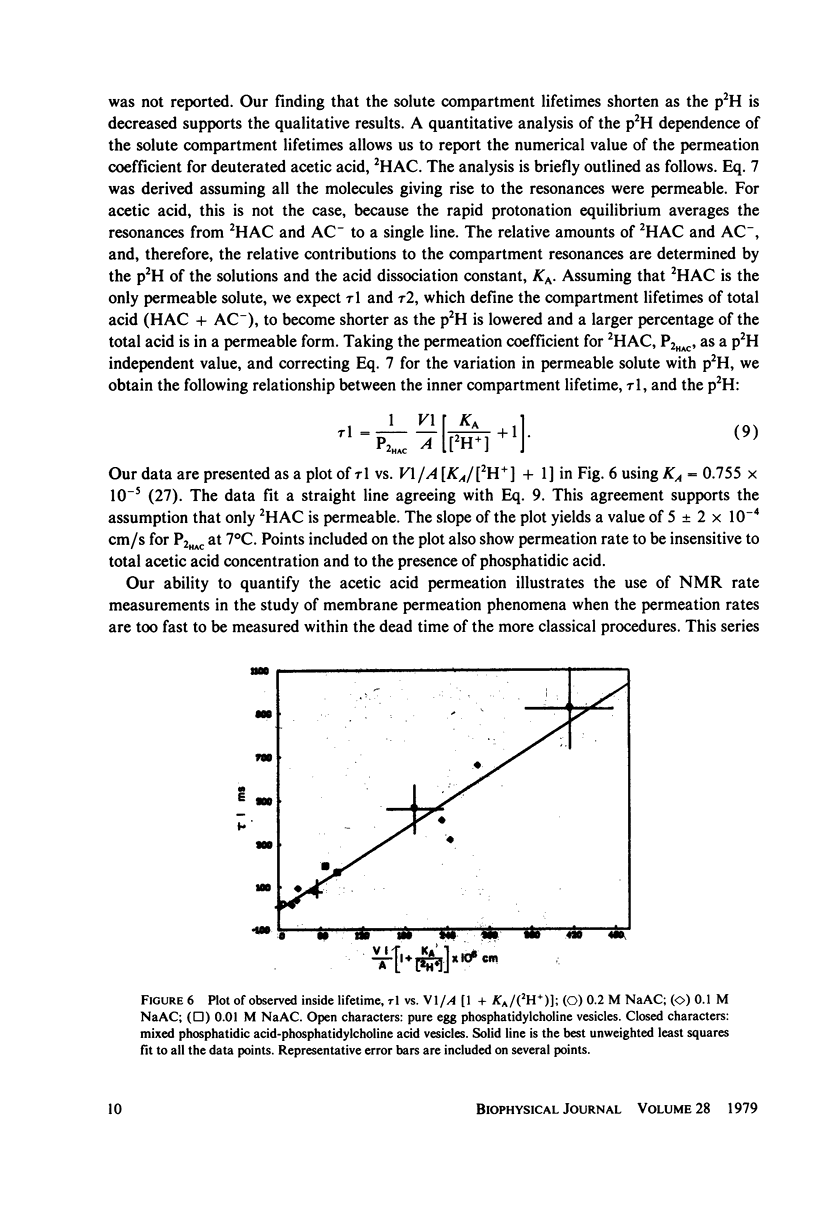
The permeation of acetic acid through large unilamellar phospholipid vesicle membranes has been investigated using the unique capability of nuclear magnetic resonance to characterize flow under pseudo-equilibrium conditions. Two types of experiments have been employed: total line shape analysis and selective population transfer. These techniques are sensitive to permeation on time scales ranging form 0.001 to 10.0 s. The permeation rate dependence on pH and acetic acid concentration indicates that the neutral acetic acid monomer is the dominant permeant species with a permeation coefficient of 5 +/- 2 x 10-4 cm/s. Mechanisms of permeation and the applicability of nuclear magnetic resonance methodology are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreoli T. E., Troutman S. L. An analysis of unstirred layers in series with "tight" and "porous" lipid bilayer membranes. J Gen Physiol. 1971 Apr;57(4):464–478. doi: 10.1085/jgp.57.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Cramer J. A., Prestegard J. H. NMR studies of pH-induced transport of carboxylic acids across phospholipid vesicle membranes. Biochem Biophys Res Commun. 1977 Mar 21;75(2):295–301. doi: 10.1016/0006-291x(77)91042-7. [DOI] [PubMed] [Google Scholar]
- Deamer D., Bangham A. D. Large volume liposomes by an ether vaporization method. Biochim Biophys Acta. 1976 Sep 7;443(3):629–634. doi: 10.1016/0005-2736(76)90483-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Carlson N. J., Scarpa A. deltapH and catecholamine distribution in isolated chromaffin granules. J Biol Chem. 1978 Mar 10;253(5):1512–1521. [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Singer M. A., Bangham A. D. The consequences of inducing salt permeability in liposomes. Biochim Biophys Acta. 1971 Aug 13;241(2):687–692. doi: 10.1016/0005-2736(71)90068-x. [DOI] [PubMed] [Google Scholar]
- Singer M. A. Transfer of anions across phospholipid membranes. Can J Physiol Pharmacol. 1973 Jul;51(7):523–531. doi: 10.1139/y73-077. [DOI] [PubMed] [Google Scholar]


