Abstract
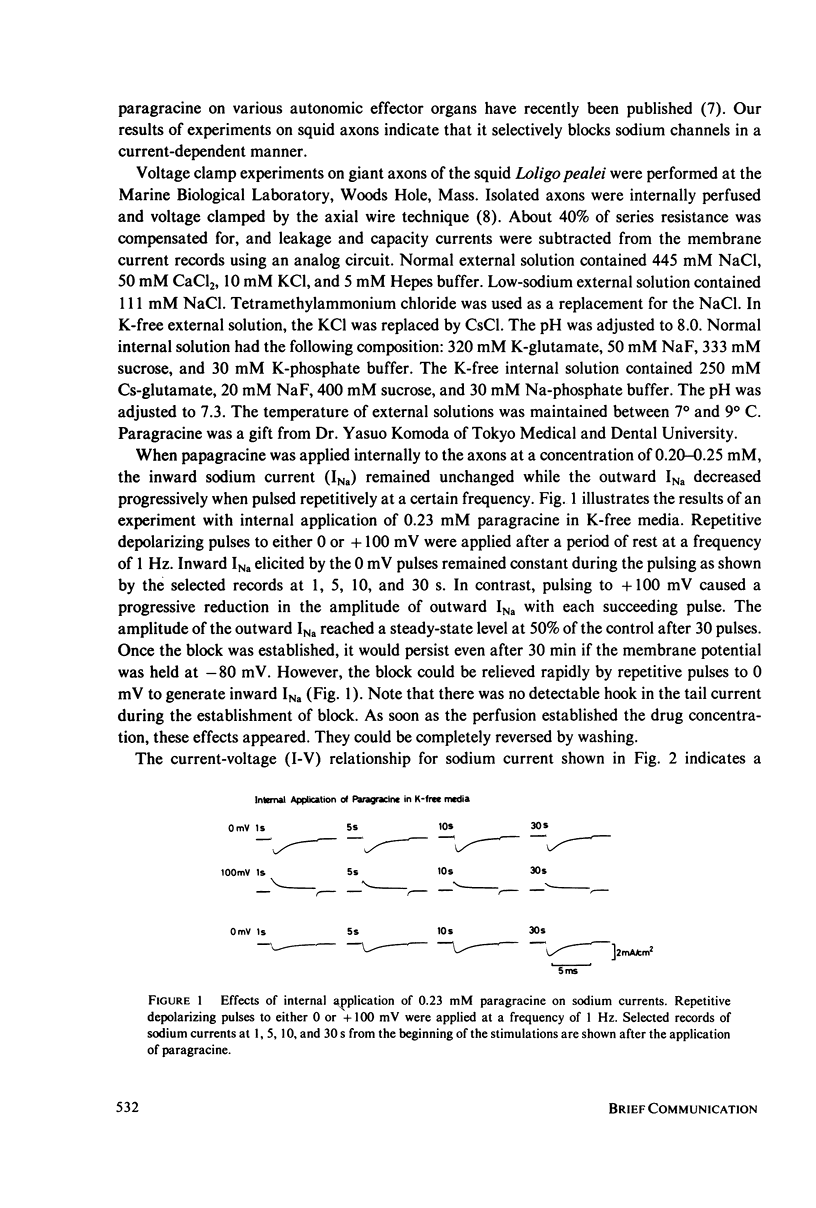
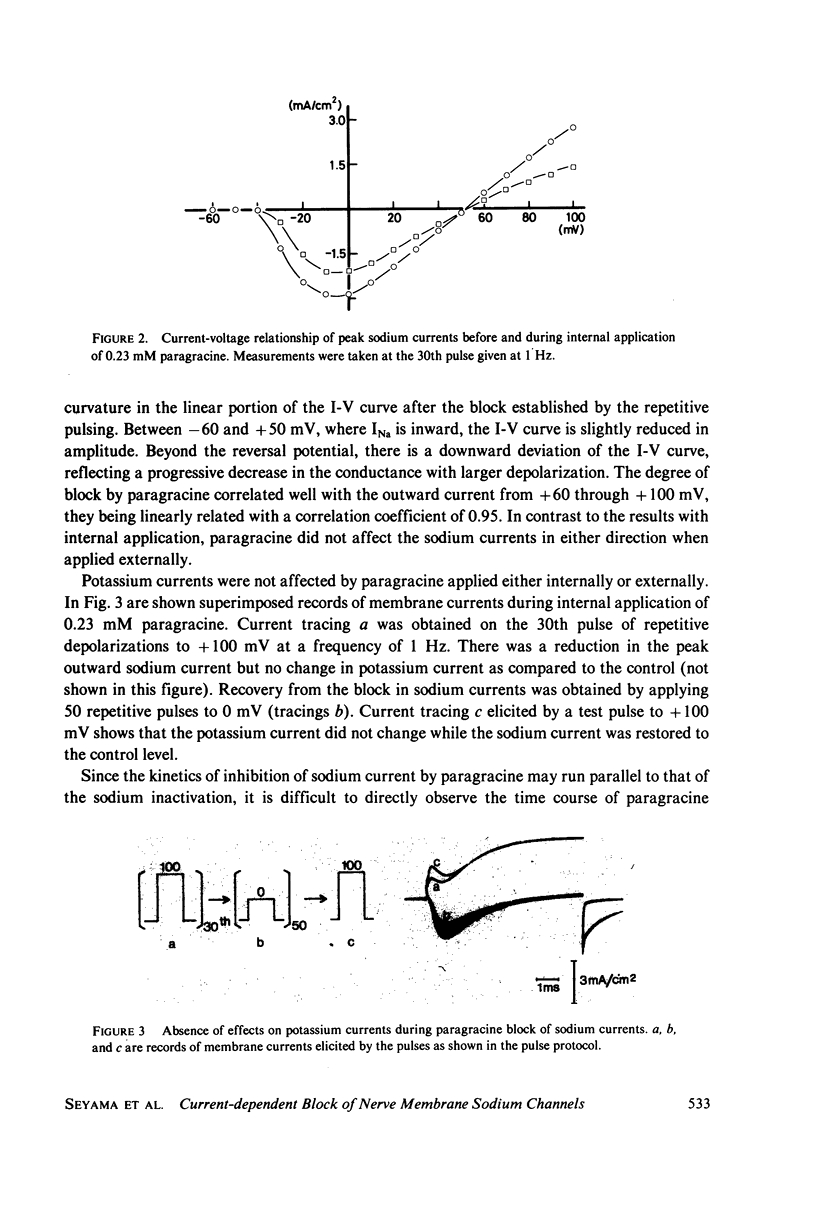
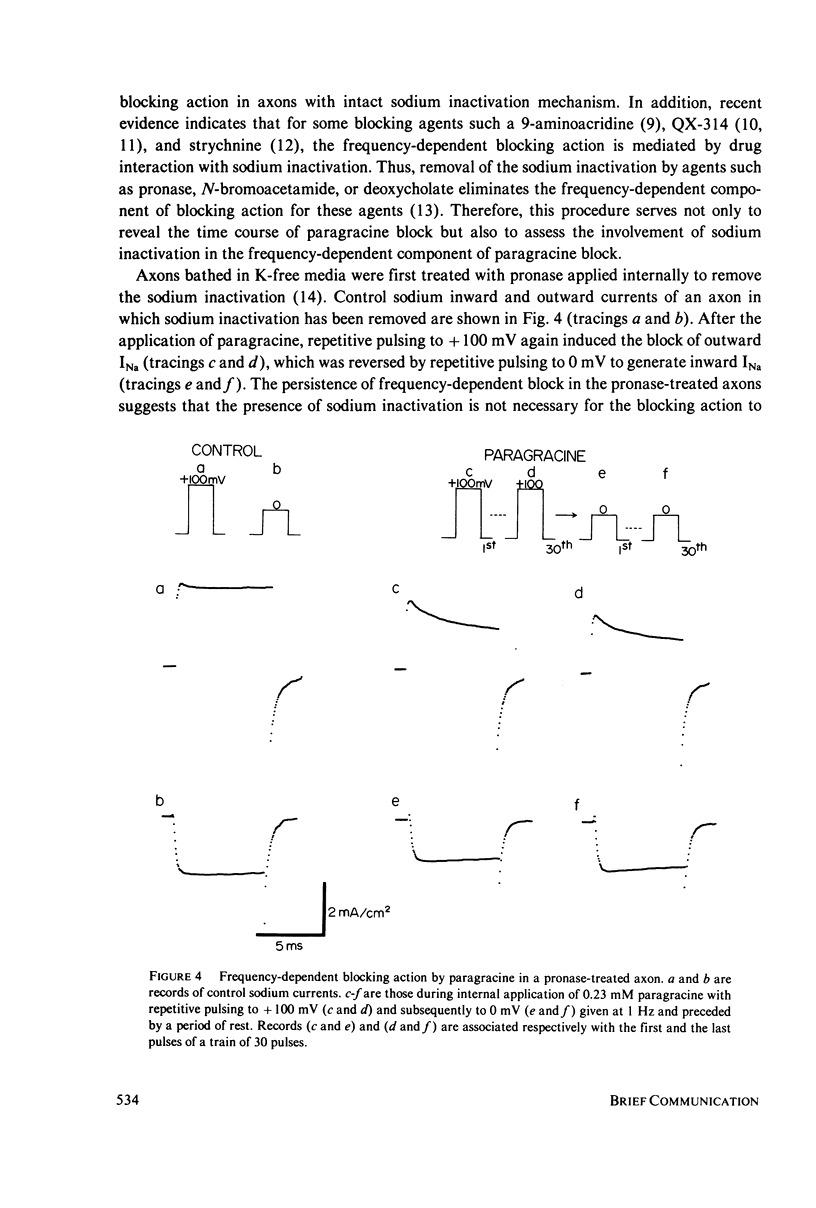
Paragracine, isolated from the coelenterate species Parazoanthus gracilis, selectively blocks sodium channels of squid axon membranes in a frequency-dependent manner. The blocking action depends on the direction and magnitude of the sodium current rather than on the absolute value of the membrane potential. Paragracine blocks the channels only from the axoplasmic side and does so only when the current is in the outward direction. This block may be reversed by generating inward sodium currents. In axons in which sodium inactivation has been removed by pronase, the frequency-dependent block persists, and a slow time-dependent block is observed. A slow interaction with its binding site in the channel may account for the frequency-dependent block.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D., Almers W. Block of sodium conductance and gating current in squid giant axons poisoned with quaternary strychnine. Biophys J. 1979 Jul;27(1):57–73. doi: 10.1016/S0006-3495(79)85202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J. M., Cohen J. B. Effect of palytoxin on membrane and potential and current of frog myelinated fibers. J Pharmacol Exp Ther. 1977 Apr;201(1):148–145. [PubMed] [Google Scholar]
- Gage P. W., Moore J. W., Westerfield M. An octopus toxin, maculotoxin, selectively blocks sodium current in squid axons. J Physiol. 1976 Jul;259(2):427–443. doi: 10.1113/jphysiol.1976.sp011474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. I. Effects of strychnine on the sodium conductance of the frog node of Ranvier. J Gen Physiol. 1977 Jun;69(6):915–926. doi: 10.1085/jgp.69.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M., Moore J. W., Kim Y. S., Padilla G. M. How Gymnodinium breve red tide toxin(s) produces repetitive firing in squid axons. Am J Physiol. 1977 Jan;232(1):C23–C29. doi: 10.1152/ajpcell.1977.232.1.C23. [DOI] [PubMed] [Google Scholar]
- Wu C. H., Narahashi T. Mechanism of action of propranolol on squid axon membranes. J Pharmacol Exp Ther. 1973 Jan;184(1):155–162. [PubMed] [Google Scholar]
- Yeh J. Z. Dynamics of 9-aminoacridine block of sodium channels in squid axons. J Gen Physiol. 1979 Jan;73(1):1–21. doi: 10.1085/jgp.73.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J. Z. Sodium inactivation mechanism modulates QX-314 block of sodium channels in squid axons. Biophys J. 1978 Nov;24(2):569–574. doi: 10.1016/S0006-3495(78)85403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


