Abstract
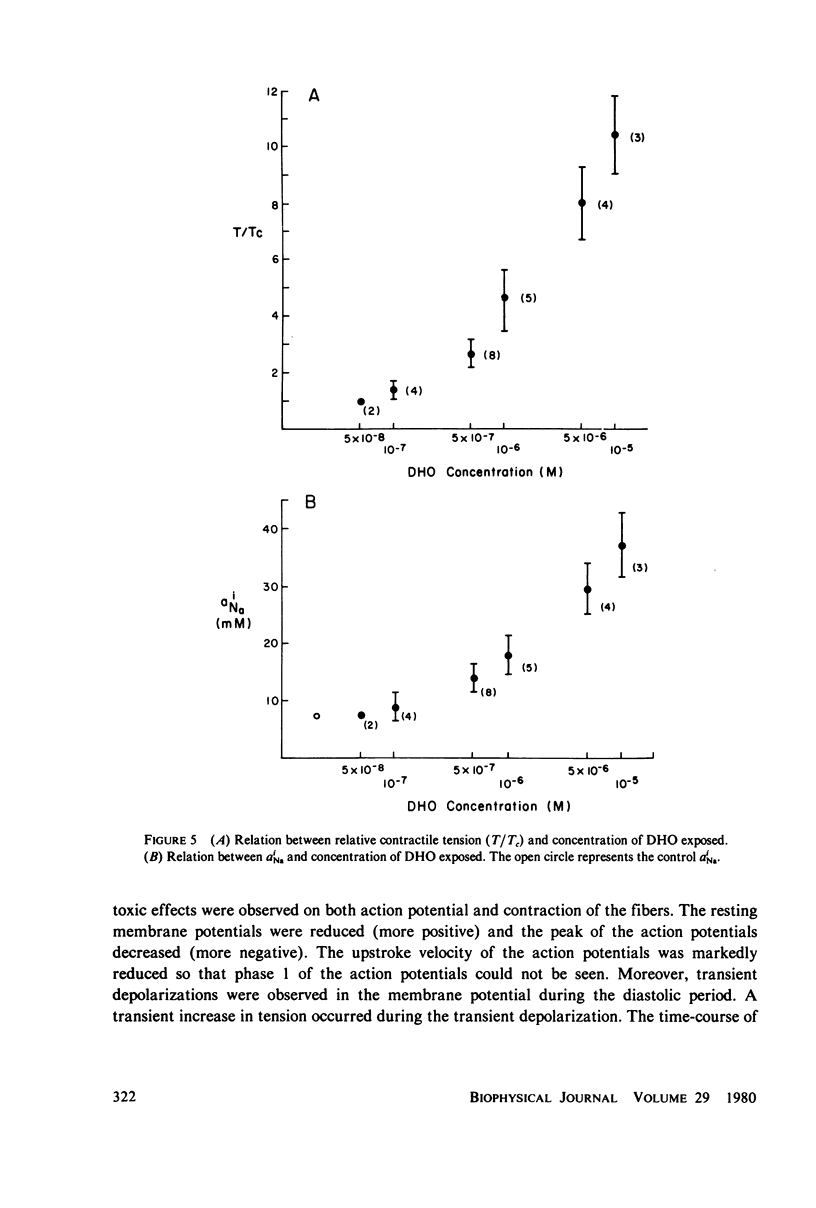
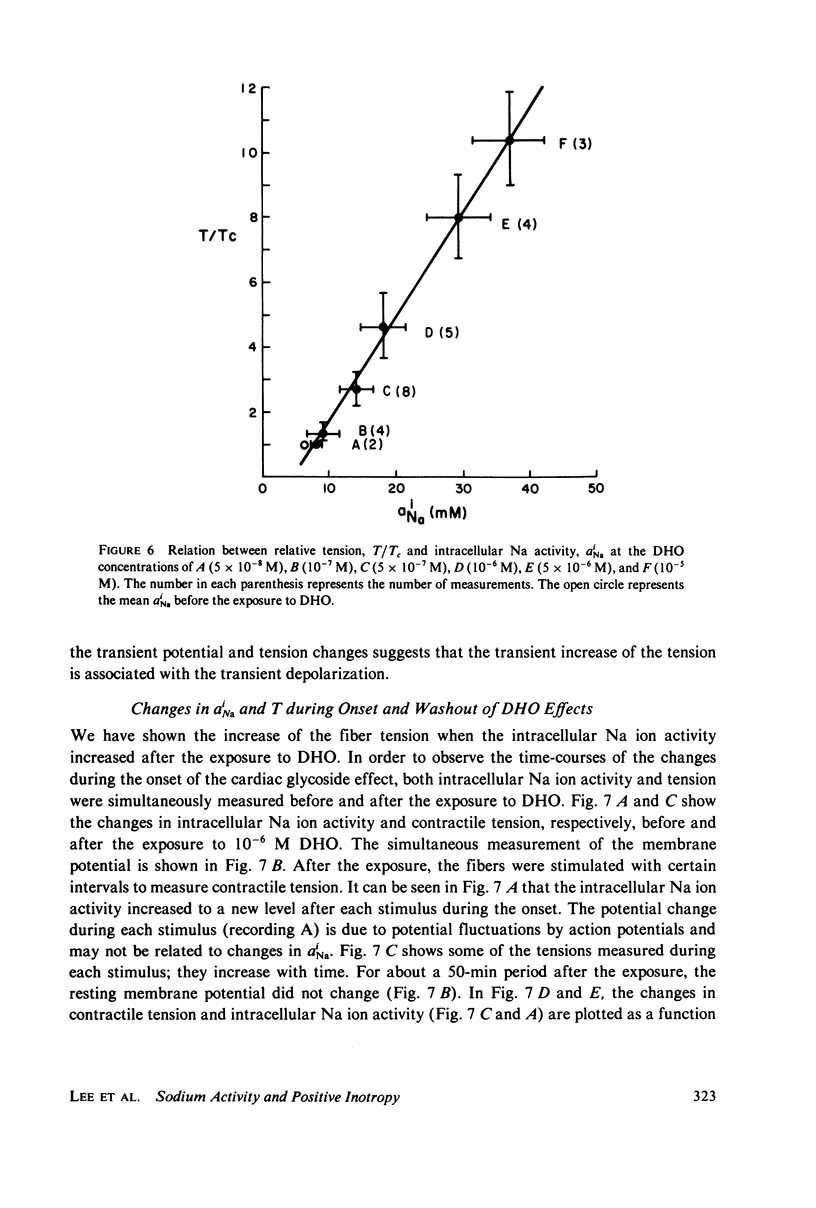
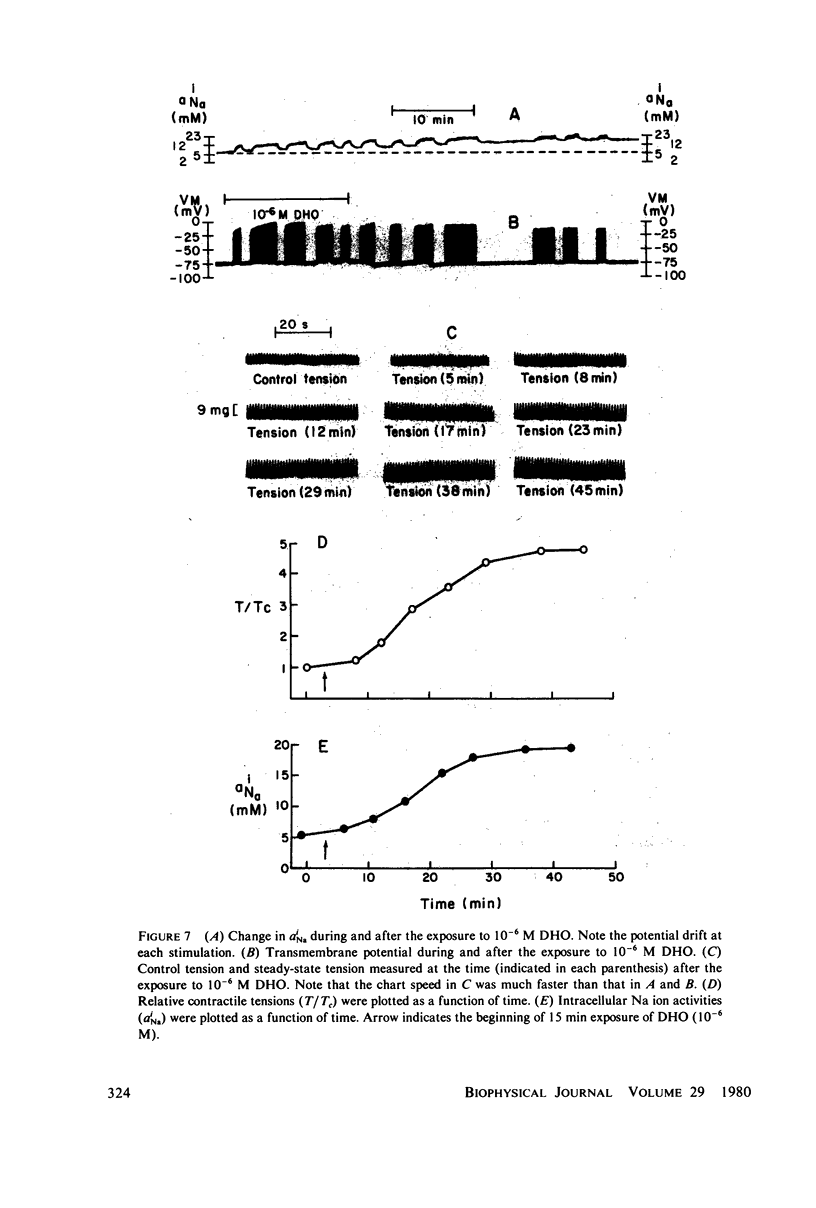
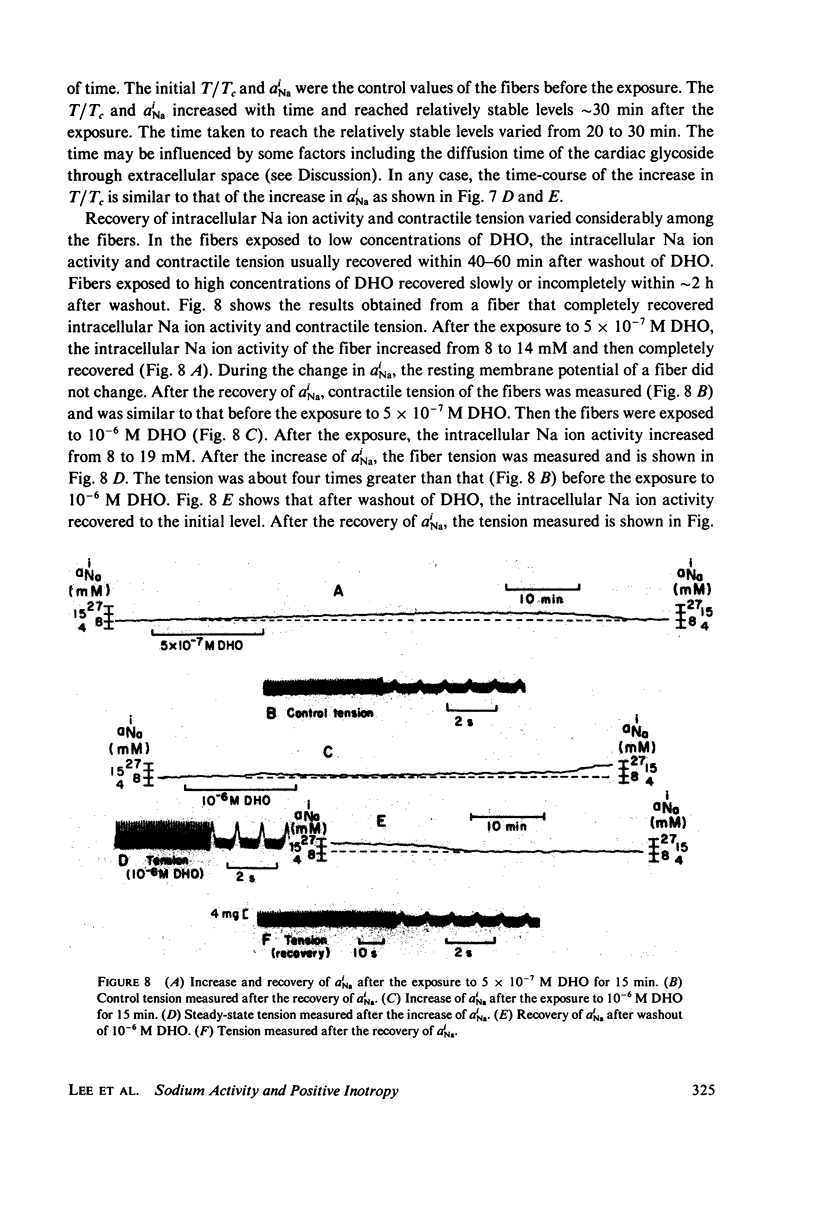
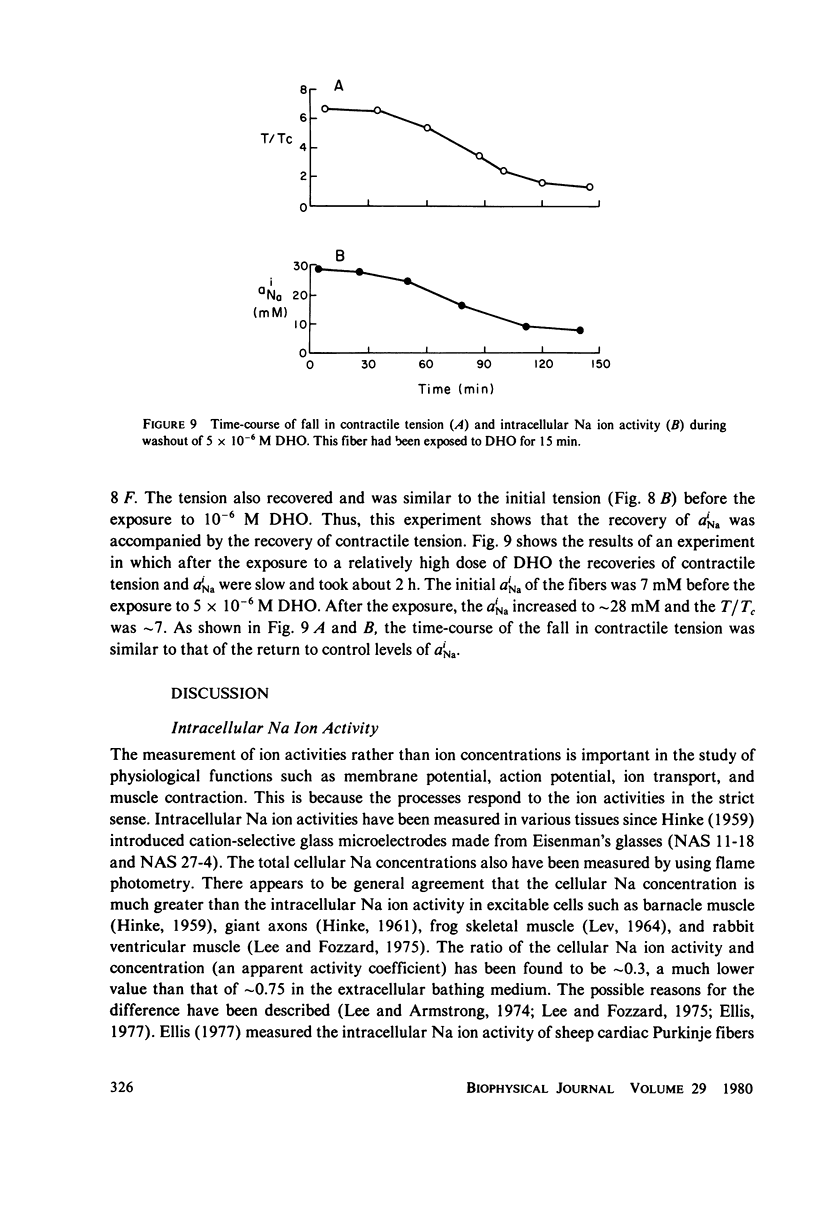
The intracellular Na ion activity (aiNa) and the contractile tension (T) of sheep cardiac Purkinje fibers were simultaneously measured employing recessed-tip Na+-selective glass microelectrodes and a mechano-electric transducer. The aiNa of 6.4 +/- 1.6 mM (mean +/- SD, n = 56) was obtained in fibers perfused with normal Tyrode's solution. The changes in aiNa and T were measured during and after the exposure of fibers to a cardiac glycoside, dihydro-ouabain (DHO) in concentrations between 5 X 10(-8) M and 10(-5) M. The exposure time to DHO was 15 min. Both aiNa and T did not change in fibers exposed to 5 X 10(-8) M DHO, and the threshold concentration for the effect of DHO appeared to be around 10(-7) M. In DHO concentrations greater than the threshold, the increases in aiNa and T strongly correlated during the onset of DHO effects. The recoveries of aiNa and T were variable and slow, being dependent on the DHO concentration. In those fibers which recovered from the effects of DHO, the time-course of aiNa recovery was similar to that of T recovery. In fibers exposed to DHO of 5 X 10(-6) M or greater, the apparent toxic effects were observed in both action potential and contraction after an initial increase in T. The fibers manifesting the apparent toxic effects has a aiNa of approximately 30 mM or greater. The results of this study indicate that the increase in aiNa is associated with the positive inotropic action of the cardiac glycoside.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akera T., Baskin S. I., Tobin T., Brody T. M. Ouabain: temporal relationship between the inotropic effect and the in vitro binding to, and dissociation from, (Na + + K + )- activated ATPase. Naunyn Schmiedebergs Arch Pharmacol. 1973;277(2):151–162. doi: 10.1007/BF00501156. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr Ionic currents in cardiac muscle: a framework for glycoside action. Fed Proc. 1977 Aug;36(9):2209–2213. [PubMed] [Google Scholar]
- Bentfeld M., Lüllmann H., Peters T., Proppe D. Interdependence of ion transport and the action of quabain in heart muscle. Br J Pharmacol. 1977 Sep;61(1):19–27. doi: 10.1111/j.1476-5381.1977.tb09735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosteels S., Carmeliet E. Estimation of intracellular Na concentration and transmembrane Na flux in cardiac Purkyne fibres. Pflugers Arch. 1972;336(1):35–47. doi: 10.1007/BF00589140. [DOI] [PubMed] [Google Scholar]
- Brody T. M., Akera T. Relations among Na+,K+-ATPase activity, sodium pump activity, transmembrane sodium movement, and cardiac contractility. Fed Proc. 1977 Aug;36(9):2219–2224. [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier G. R., Saunders J. H., Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973 May;32(5):600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., Lee C. O. Influence of changes in external potassium and chloride ions on membrane potential and intracellular potassium ion activity in rabbit ventricular muscle. J Physiol. 1976 Apr;256(3):663–689. doi: 10.1113/jphysiol.1976.sp011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. THE ACTION OF CARDIAC GLYCOSIDES ON ION MOVEMENTS. Pharmacol Rev. 1964 Dec;16:381–407. [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Voltage dependence and time dependence of contraction in sheep cardiac Purkinje fibers. Circ Res. 1971 Apr;28(4):446–460. doi: 10.1161/01.res.28.4.446. [DOI] [PubMed] [Google Scholar]
- HAJDU S., LEONARD E. The cellular basis of cardiac glycoside action. Pharmacol Rev. 1959 Jun;11(2 Pt 1):173–209. [PubMed] [Google Scholar]
- HINKE J. A. Glass micro-electrodes for measuring intracellular activities of sodium and potassium. Nature. 1959 Oct 17;184(Suppl 16):1257–1258. doi: 10.1038/1841257a0. [DOI] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen T. J., Smith T. W. Inhibition of myocardial monovalent cation active transport by subtoxic doses of ouabain in the dog. Circ Res. 1978 Jun;42(6):856–863. doi: 10.1161/01.res.42.6.856. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEV A. A. DETERMINATION OF ACTIVITY AND ACTIVITY COEFFICIENTS OF POTASSIUM AND SODIUM IONS IN FROG MUSCLE FIBRES. Nature. 1964 Mar 14;201:1132–1134. doi: 10.1038/2011132a0. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Relationship between myocardial contractility and the effects of digitalis on ionic exchange. Fed Proc. 1977 Aug;36(9):2231–2234. [PubMed] [Google Scholar]
- Lee C. O., Armstrong W. M. State and distribution of potassium and sodium ions in frog skeletal muscle. J Membr Biol. 1974;15(4):331–362. doi: 10.1007/BF01870094. [DOI] [PubMed] [Google Scholar]
- Lee C. O. Electrochemical properties of Na+- and K+-selective glass microelectrodes. Biophys J. 1979 Aug;27(2):209–220. doi: 10.1016/S0006-3495(79)85212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Klaus W. The subcellular basis for the mechanism of inotropic action of cardiac glycosides. Pharmacol Rev. 1971 Sep;23(3):193–261. [PubMed] [Google Scholar]
- Miura D. S., Rosen M. R. The effects of ouabain on the transmembrane potentials and intracellular potassium activity of canine cardiac Purkinje fibers. Circ Res. 1978 Mar;42(3):333–338. doi: 10.1161/01.res.42.3.333. [DOI] [PubMed] [Google Scholar]
- Okita G. T. Dissociation of Na+,K+-ATPase inhibition from digitalis inotropy. Fed Proc. 1977 Aug;36(9):2225–2230. [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Merker C., Hoffman B. F. Mechanisms of digitalis toxicity. Effects of ouabain on phase four of canine Purkinje fiber transmembrane potentials. Circulation. 1973 Apr;47(4):681–689. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Allen J. C., Van Winkle W. B., Munson R. Further studies on the correlation between the inotropic action of ouabain and its interaction with the Na+,K+-adenosine triphosphatase: isolated perfused rabbit and cat hearts. J Pharmacol Exp Ther. 1974 Oct;191(1):119–127. [PubMed] [Google Scholar]
- Shlafer M., Somani P., Pressman B. C., Palmer R. F. Effects of carboxylic ionophore monensin on atrial contractility and Ca2+ regulation by isolated cardiac microsomes. J Mol Cell Cardiol. 1978 Apr;10(4):333–346. doi: 10.1016/0022-2828(78)90382-6. [DOI] [PubMed] [Google Scholar]
- Sutko J. L., Besch H. R., Jr, Bailey J. C., Zimmerman G., Watanabe A. M. Direct effects of the monovalent cation ionophores monensin and nigericin on myocardium. J Pharmacol Exp Ther. 1977 Dec;203(3):685–700. [PubMed] [Google Scholar]
- Wallick E. T., Lindenmayer G. E., Lane L. K., Allen J. C., Pitts B. J., Schwartz A. Recent advances in cardiac glycoside-Na+,K+-ATPase interaction. Fed Proc. 1977 Aug;36(9):2214–2218. [PubMed] [Google Scholar]


