Abstract
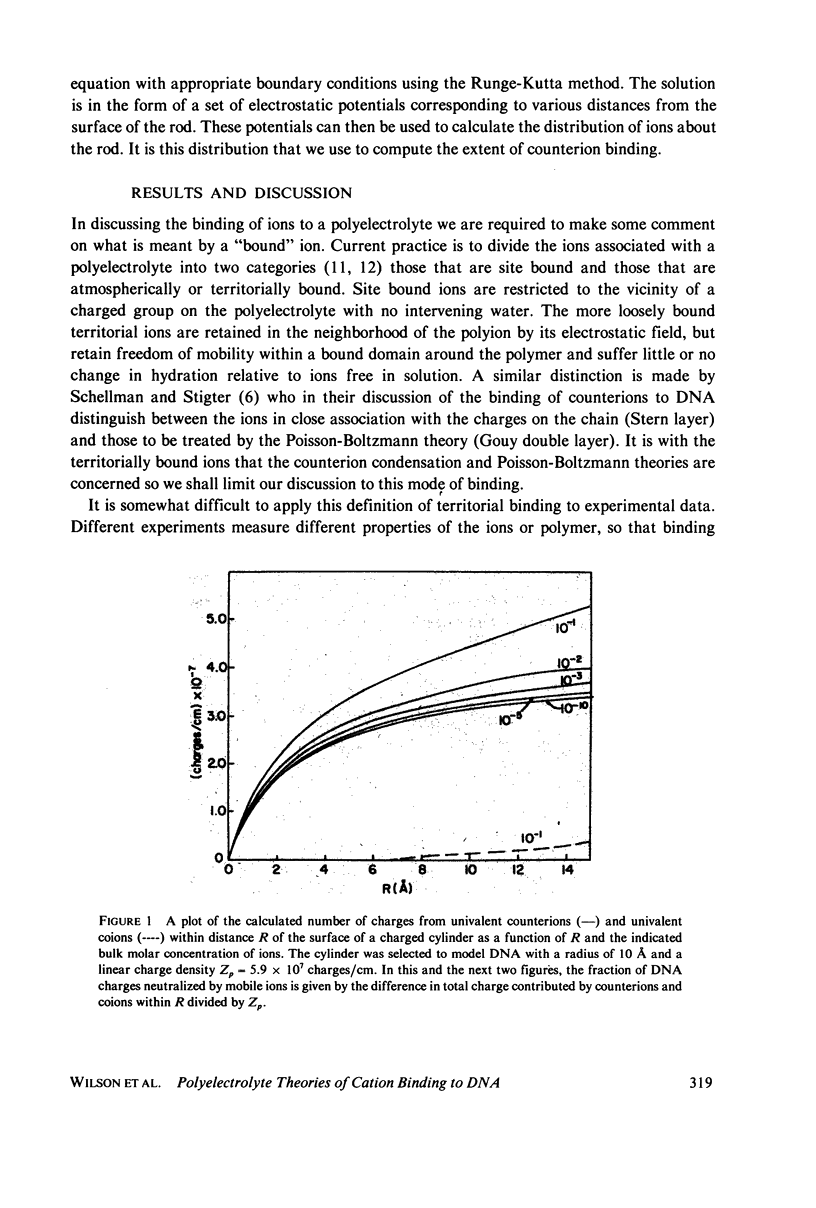
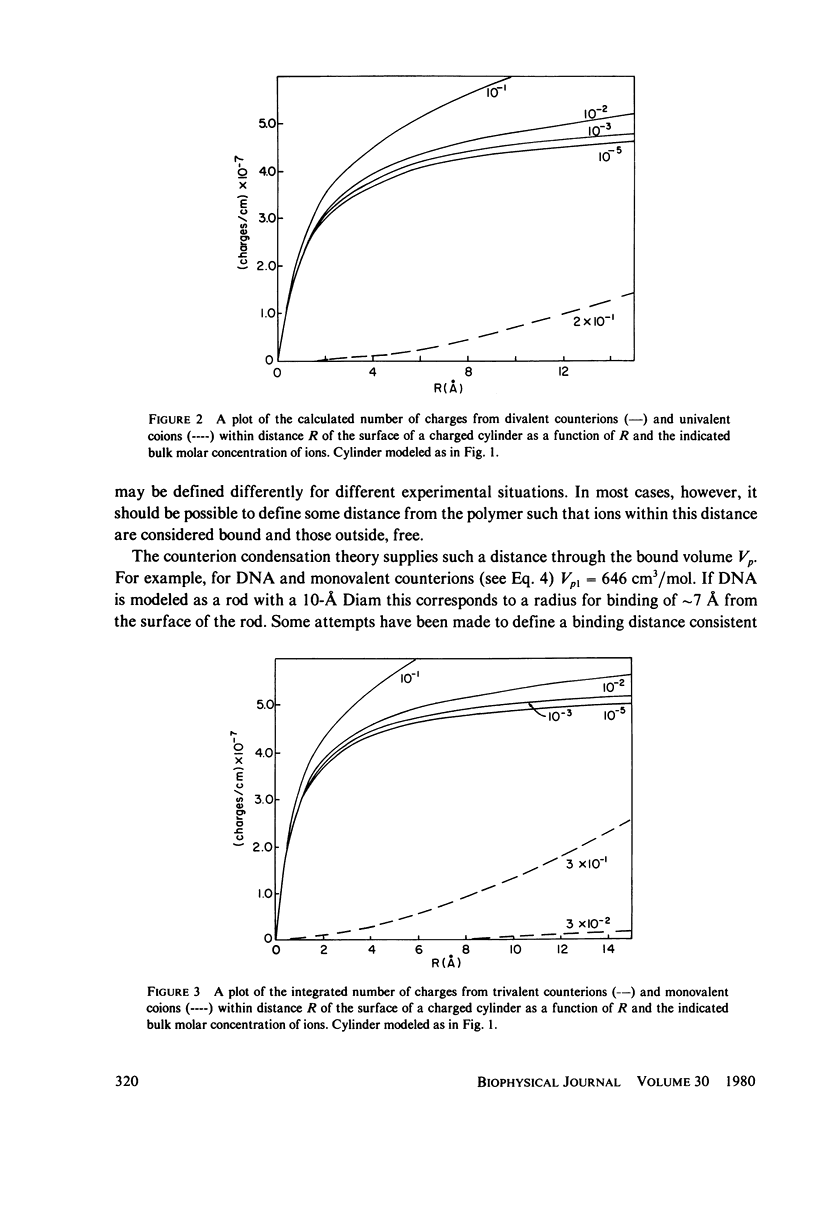
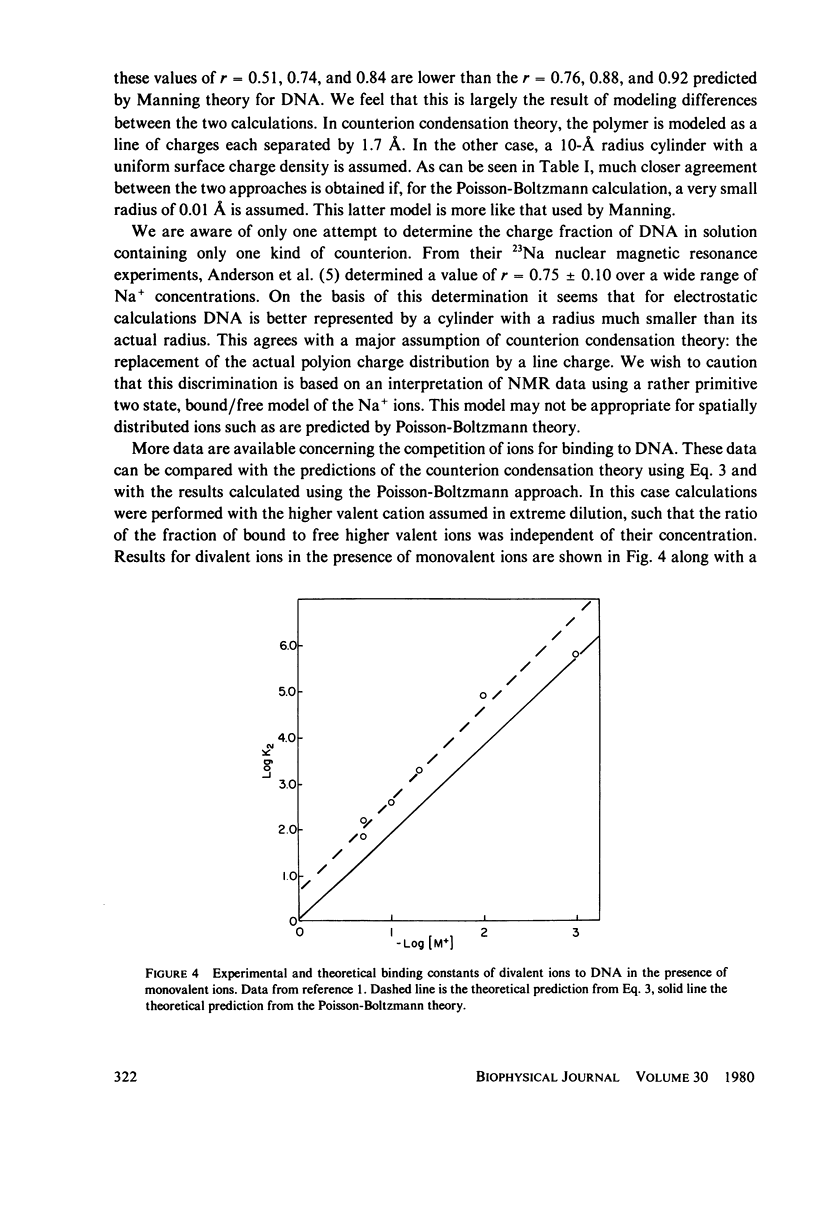
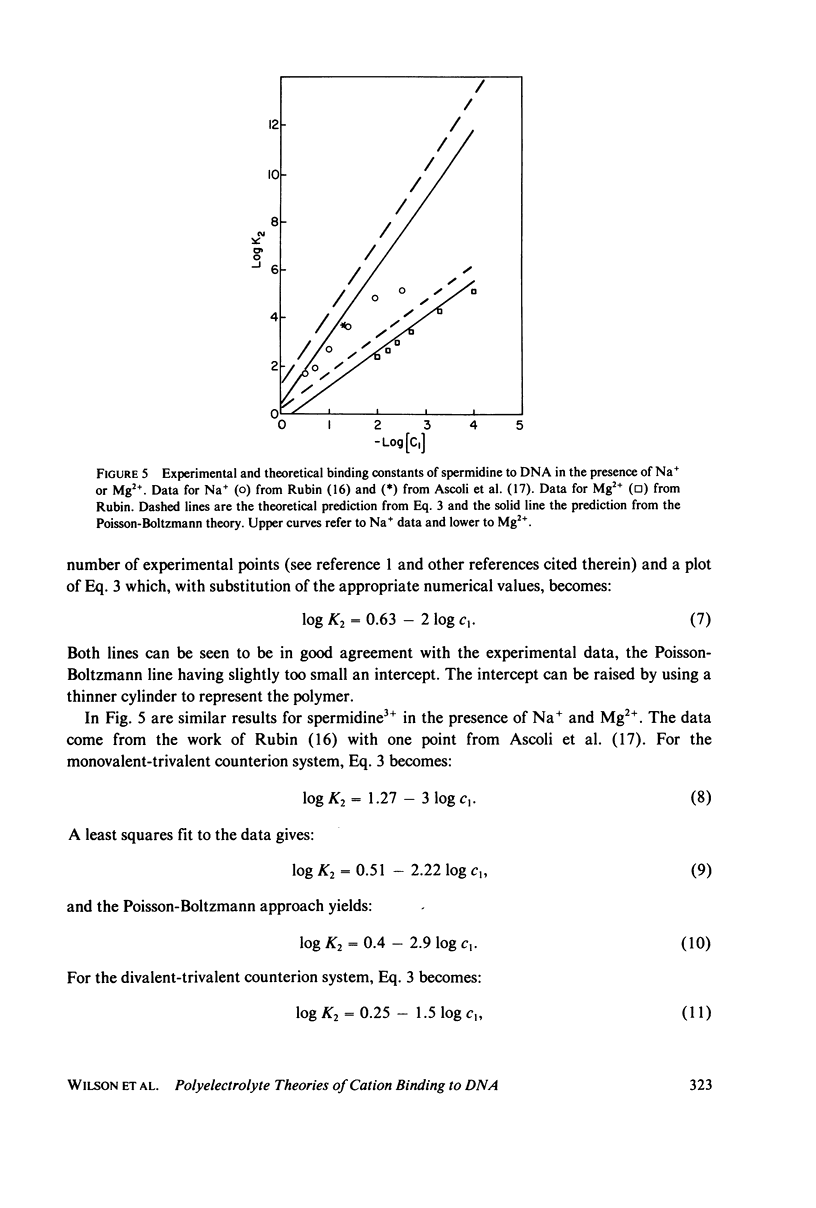
Predictions of the binding of counterions to DNA made using the counterion condensation theory developed by Manning are compared with those made using the Poisson-Boltzmann equation, solved numerically by the Runge-Kutta procedure. Ions are defined as territorially or atmospherically bound if they fall within a given distance, defined by counterion condensation theory, from the DNA surface. Two types of experimental situations are considered. The first is the delocalized binding of a single type of counterion to DNA. In this case the Poisson-Boltzmann treatment predicts somewhat lower extents of binding TO DNA, modeled as a 10-A radius cylinder, than does Manning theory. The two theories converge as the radius decreases. The second type of experiment is the competition of ions of different valence for binding to DNA. The theories are compared with literature values of binding constants of divalent ions in the presence of monovalent ions, and of spermidine 3+ in the presence of Na+ or Mg2+. Both predict with fair accuracy the salt dependence of the equilibrium constants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. F., Record M. T., Jr, Hart P. A. Sodium-23 NMR studies of cation-DNA interactions. Biophys Chem. 1978 Jan;7(4):301–316. doi: 10.1016/0301-4622(78)85007-8. [DOI] [PubMed] [Google Scholar]
- Manning G. S. Limiting laws and counterion condensation in polyelectrolyte solutions. IV. The approach to the limit and the extraordinary stability of the charge fraction. Biophys Chem. 1977 Sep;7(2):95–102. doi: 10.1016/0301-4622(77)80002-1. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, Lohman M. L., De Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976 Oct 25;107(2):145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Record M. T., Jr, deHaseth P. L., Lohman T. M. Interpretation of monovalent and divalent cation effects on the lac repressor-operator interaction. Biochemistry. 1977 Nov 1;16(22):4791–4796. doi: 10.1021/bi00641a005. [DOI] [PubMed] [Google Scholar]
- Rubin R. L. Spermidine-Deoxyribonucleic acid interaction in vitro and in Escherichia coli. J Bacteriol. 1977 Feb;129(2):916–925. doi: 10.1128/jb.129.2.916-925.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellman J. A. Electrical double layer, zeta potential, and electrophoretic charge of double-stranded DNA. Biopolymers. 1977 Jul;16(7):1415–1434. doi: 10.1002/bip.1977.360160704. [DOI] [PubMed] [Google Scholar]
- Spegt P., Weill G. Magnetic resonance distinction between site bound and atmospherically bound paramagnetic counterions in polyelectrolyte solutions. Biophys Chem. 1976 Mar;4(2):143–149. doi: 10.1016/0301-4622(76)85004-1. [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Bloomfield V. A. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979 May 29;18(11):2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]


