Abstract
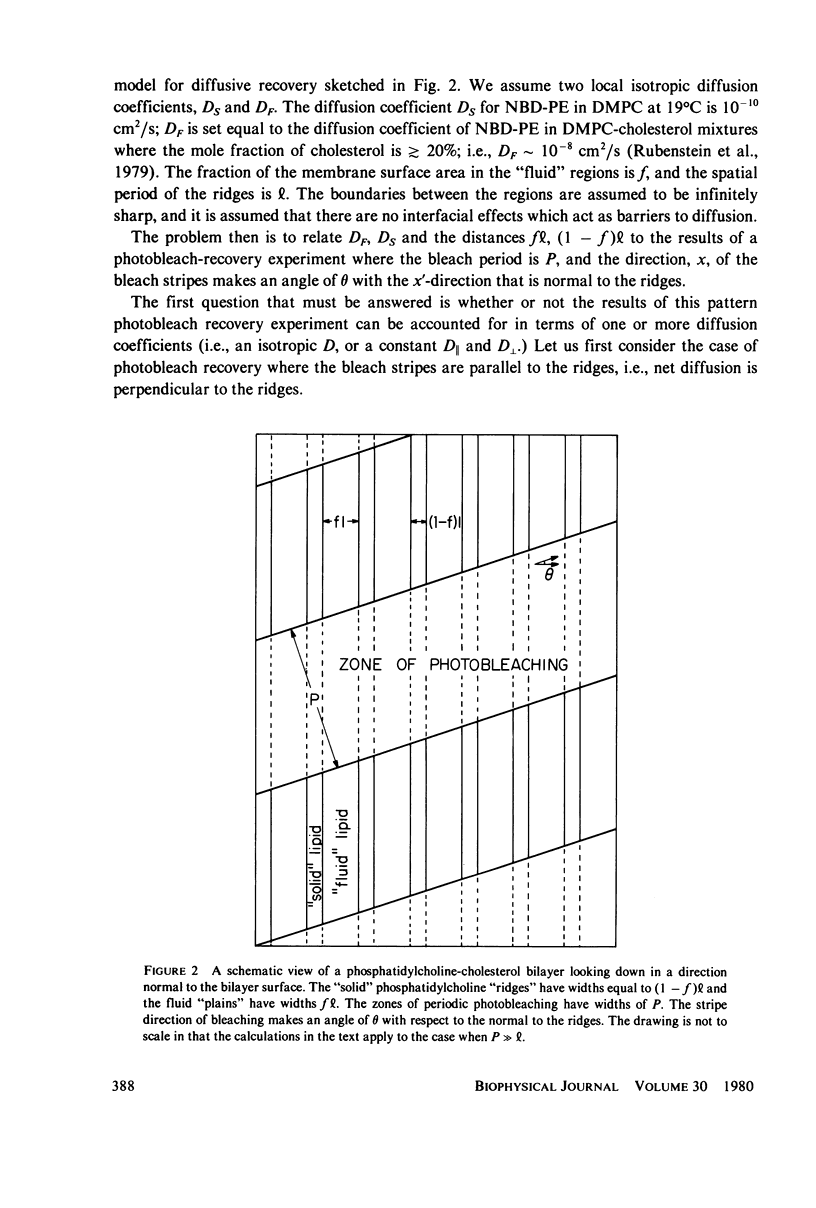
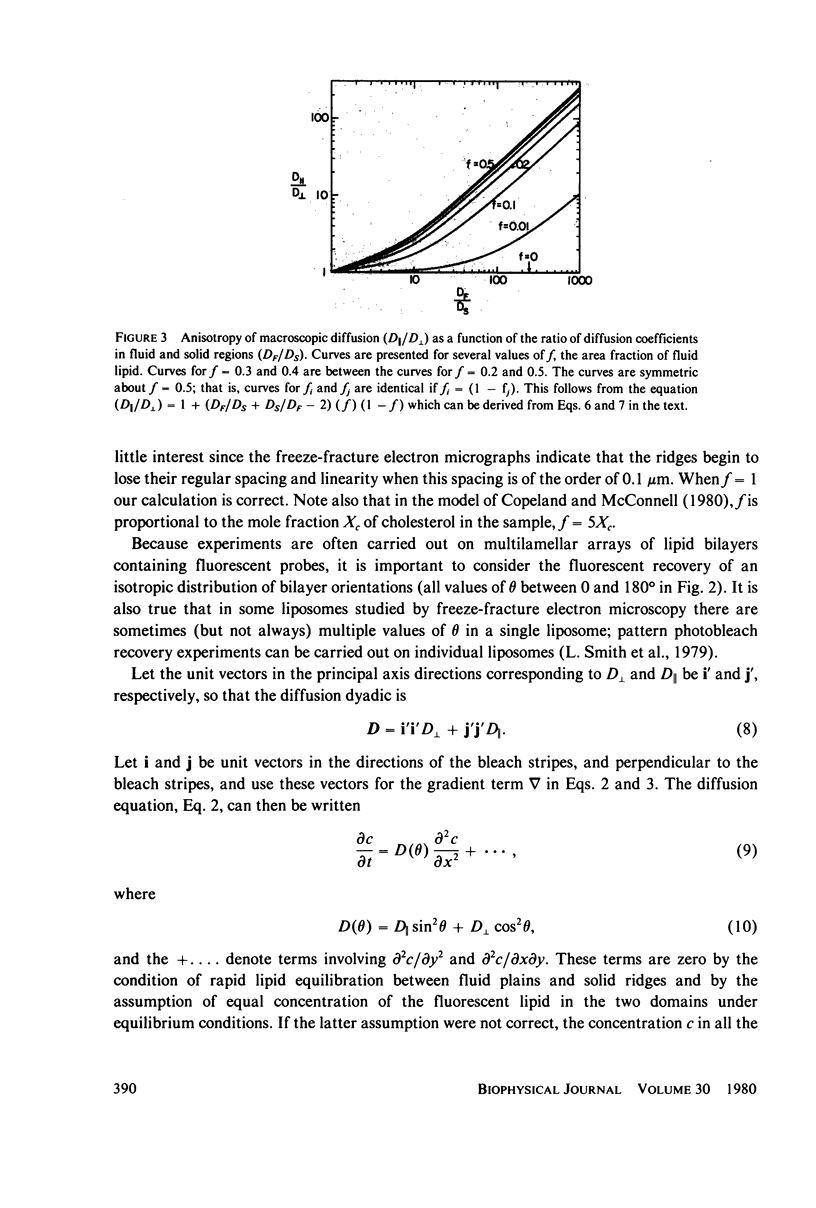
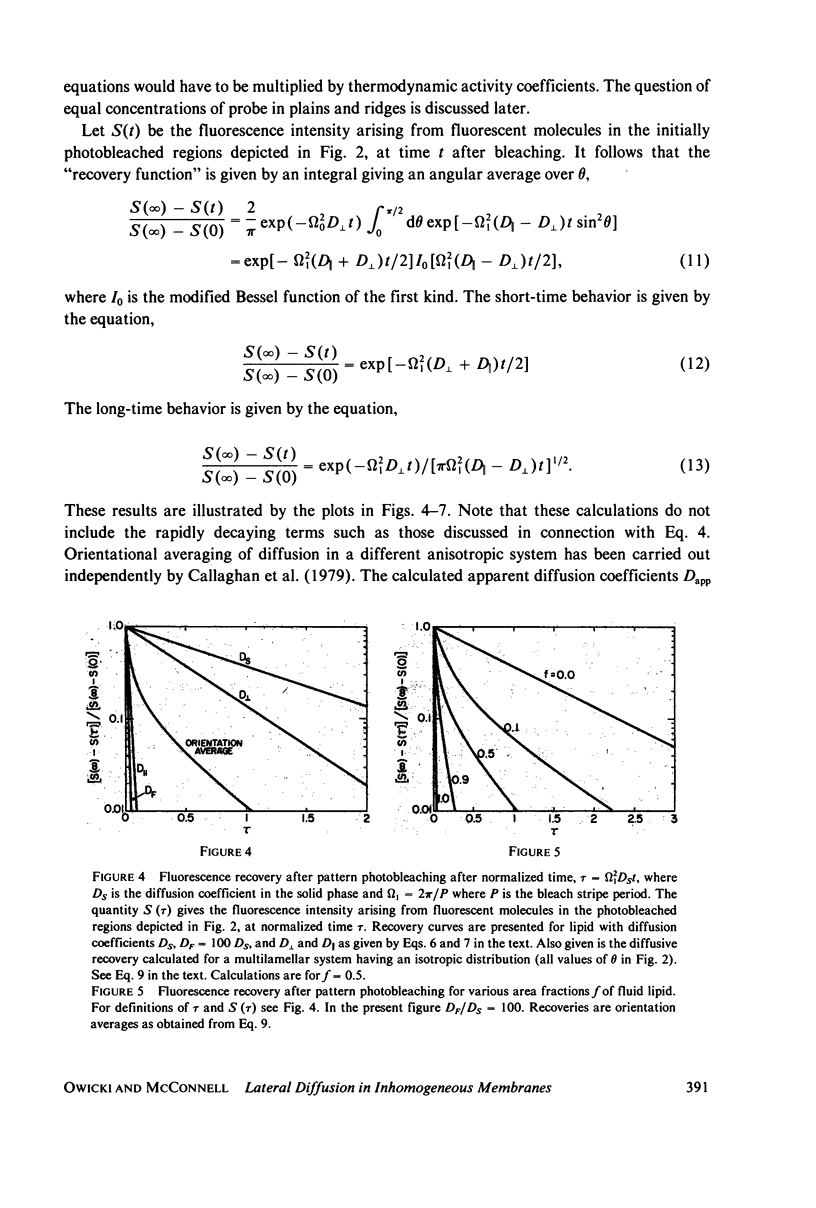
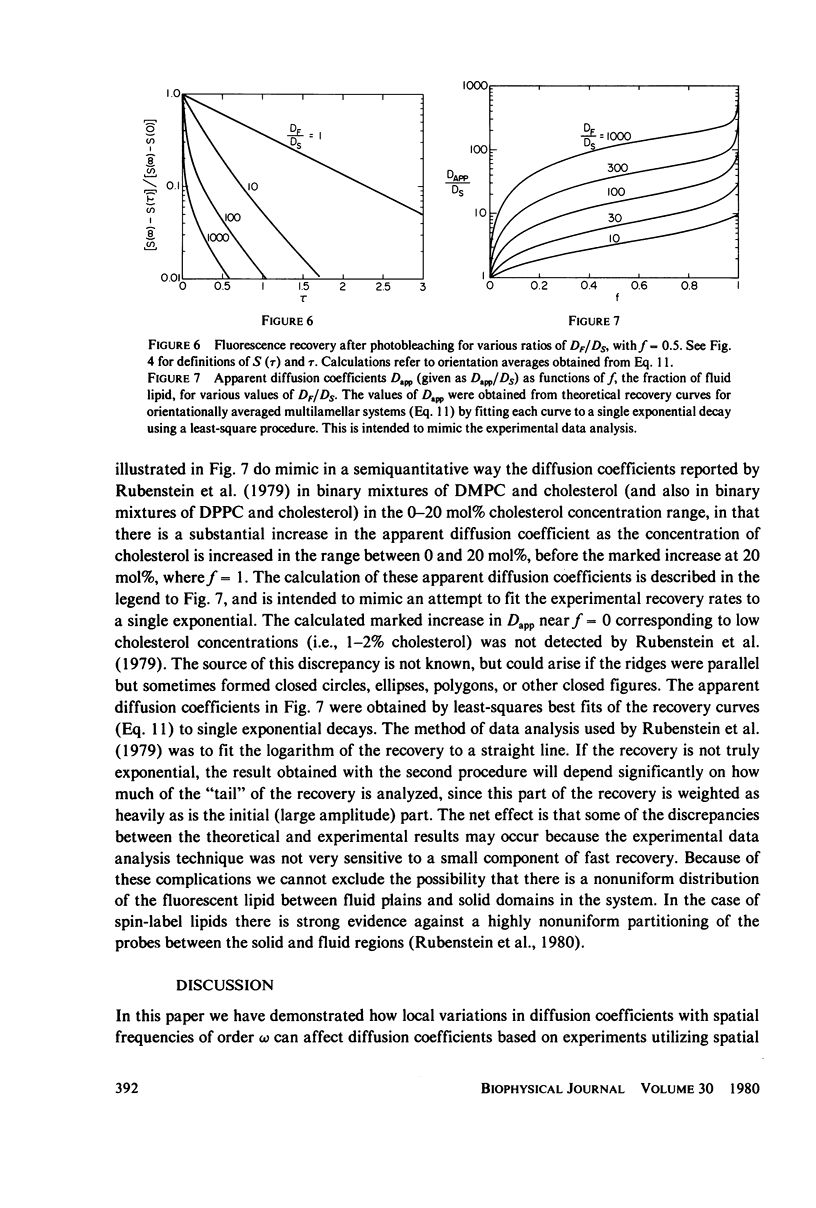
The problem of lateral diffusion in inhomogeneous membranes is illustrated by a theoretical calculation of the lateral diffusion of a fluorescent lipid probe in binary mixtures of phosphatidylcholine and cholesterol under conditions of temperature and composition such that this lipid mixture consists of alternating parallel domains of fluid and solid lipid, having separations that are small compared with the distance scale employed in photobleaching experiments. The theoretical calculations clearly illustrate how inhomogeneities in membrane composition affecting the lateral motion of membrane components on a small (10-100 nm) distance scale can give complex diffusive responses in experiments such as fluorescence photobleaching that employ comparatively macroscopic distances (10-100 micrometers) for the measurement of diffusive recovery. The theoretical calculations exhibit the unusual dependence of the apparent lateral diffusion coefficient of a fluorescent lipid probe on lipid composition in binary mixtures of cholesterol and phosphatidylcholines as reported by Rubenstein et al. (1979, Proc. Natl. Acad. Sci. U.S.A., 76:15-18).
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok M. C., Van Deenen L. L., De Gier J. The effect of cholesterol incorporation on the temperature dependence of water permeation through liposomal membranes prepared from phosphatidylcholines. Biochim Biophys Acta. 1977 Feb 4;464(3):509–518. doi: 10.1016/0005-2736(77)90026-8. [DOI] [PubMed] [Google Scholar]
- Brûlet P., McConnell H. M. Kinetics of phase equilibrium in a binary mixture of phospholipids. J Am Chem Soc. 1976 Mar 17;98(6):1314–1318. doi: 10.1021/ja00422a003. [DOI] [PubMed] [Google Scholar]
- Callaghan P. T., Jolley K. W., Lelievre J. Diffusion of water in the endosperm tissue of wheat grains as studied by pulsed field gradient nuclear magnetic resonance. Biophys J. 1979 Oct;28(1):133–141. doi: 10.1016/S0006-3495(79)85164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux P., McConnell H. M. Lateral diffusion in spin-labeled phosphatidylcholine multilayers. J Am Chem Soc. 1972 Jun 28;94(13):4475–4481. doi: 10.1021/ja00768a600. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Esser A. F., Bartholomew R. M., Parce J. W., McConnell H. M. The physical state of membrane lipids modulates the activation of the first component of complement. J Biol Chem. 1979 Mar 25;254(6):1768–1770. [PubMed] [Google Scholar]
- Estep T. N., Mountcastle D. B., Biltonen R. L., Thompson T. E. Studies on the anomalous thermotropic behavior of aqueous dispersions of dipalmitoylphosphatidylcholine-cholesterol mixtures. Biochemistry. 1978 May 16;17(10):1984–1989. doi: 10.1021/bi00603a029. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., Parce J. W., McConnell H. M. Specific antibody-dependent activation of neutrophils by liposomes containing spin-label lipid haptens. Biochem Biophys Res Commun. 1979 Feb 14;86(3):522–528. doi: 10.1016/0006-291x(79)91745-5. [DOI] [PubMed] [Google Scholar]
- Jacobs R., Oldfield E. Deuterium nuclear magnetic resonance investigation of dimyristoyllecithin--dipalmitoyllecithin and dimyristoyllecithin--cholesterol mixtures. Biochemistry. 1979 Jul 24;18(15):3280–3285. doi: 10.1021/bi00582a013. [DOI] [PubMed] [Google Scholar]
- Kleemann W., McConnell H. M. Interactions of proteins and cholesterol with lipids in bilayer membranes. Biochim Biophys Acta. 1976 Jan 21;419(2):206–222. doi: 10.1016/0005-2736(76)90347-3. [DOI] [PubMed] [Google Scholar]
- Kuo A. L., Wade C. G. Lipid lateral diffusion by pulsed nuclear magnetic resonance. Biochemistry. 1979 May 29;18(11):2300–2308. doi: 10.1021/bi00578a026. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Mateo P. L., Sturtevant J. M. High-sensitivity scanning calorimetric study of mixtures of cholesterol with dimyristoyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1978 Jun 13;17(12):2464–2468. doi: 10.1021/bi00605a034. [DOI] [PubMed] [Google Scholar]
- McConnell H. M. Relation of lateral molecular motion in membranes and immune response. Harvey Lect. 1978;72:231–252. [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Parce J. W., McConnell H. M., Bartholomew R. M., Esser A. F. Kinetics of antibody-dependent activation of the first component of complement on lipid bilayer membranes. Biochem Biophys Res Commun. 1980 Mar 13;93(1):235–242. doi: 10.1016/s0006-291x(80)80271-3. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Poo M., Cone R. A. Lateral diffusion of rhodopsin in the photoreceptor membrane. Nature. 1974 Feb 15;247(5441):438–441. doi: 10.1038/247438a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Owicki J. C., McConnell H. M. Dynamic properties of binary mixtures of phosphatidylcholines and cholesterol. Biochemistry. 1980 Feb 5;19(3):569–573. doi: 10.1021/bi00544a027. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Smith B. A., McConnell H. M. Lateral diffusion in binary mixtures of cholesterol and phosphatidylcholines. Proc Natl Acad Sci U S A. 1979 Jan;76(1):15–18. doi: 10.1073/pnas.76.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Sheats J. R., McConnell H. M. A photochemical technique for measuring lateral diffusion of spin-labeled phospholipids in membranes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4661–4663. doi: 10.1073/pnas.75.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshick E. J., McConnell H. M. Lateral phase separations in binary mixtures of cholesterol and phospholipids. Biochem Biophys Res Commun. 1973 Jul 17;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- Smith B. A., Clark W. R., McConnell H. M. Anisotropic molecular motion on cell surfaces. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5641–5644. doi: 10.1073/pnas.76.11.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., McConnell H. M. Determination of molecular motion in membranes using periodic pattern photobleaching. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2759–2763. doi: 10.1073/pnas.75.6.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I. C., Butler K. W., Tulloch A. P., Davis J. H., Bloom M. The properties of gel state lipid in membranes of Acholeplasma laidlawii as observed by 2H NMR. FEBS Lett. 1979 Apr 1;100(1):57–61. doi: 10.1016/0014-5793(79)81130-8. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Parce J. W., Smith B. A., McConnell H. M. Antibodies bound to lipid haptens in model membranes diffuse as rapidly as the lipids themselves. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4177–4179. doi: 10.1073/pnas.76.9.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Träuble H., Sackmann E. Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc. 1972 Jun 28;94(13):4499–4510. doi: 10.1021/ja00768a015. [DOI] [PubMed] [Google Scholar]


