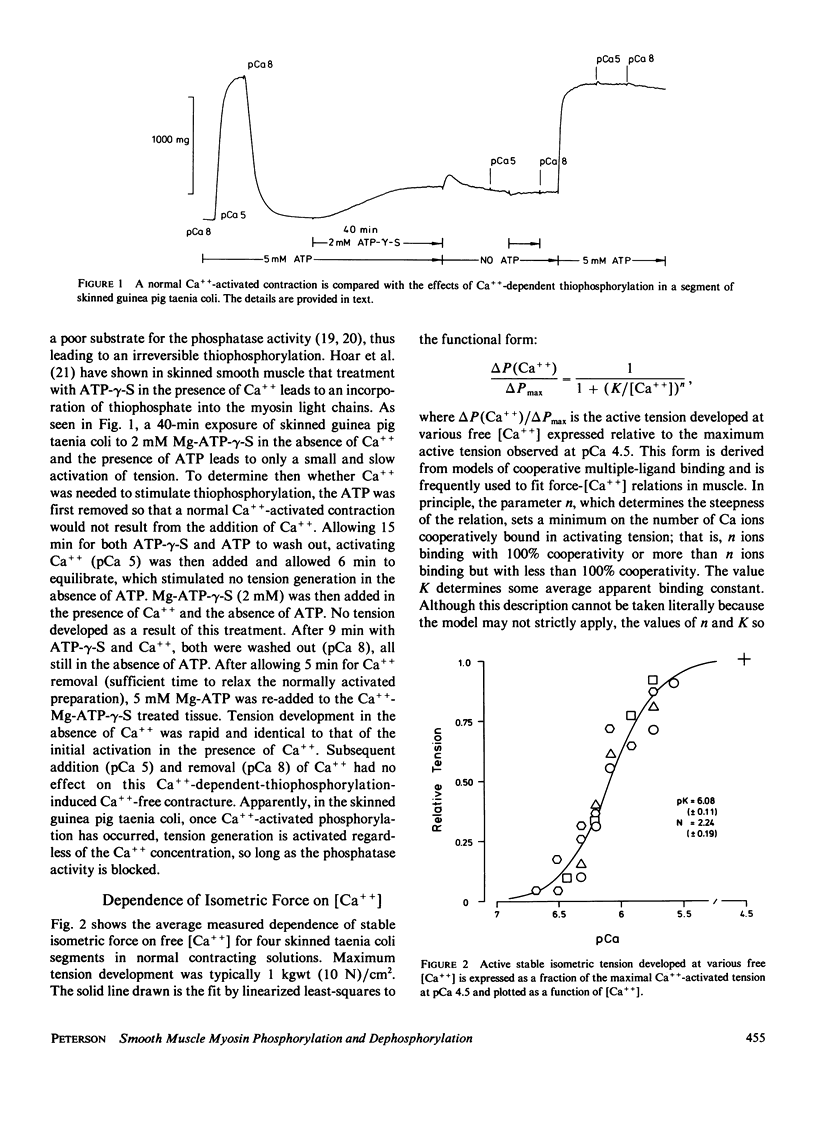
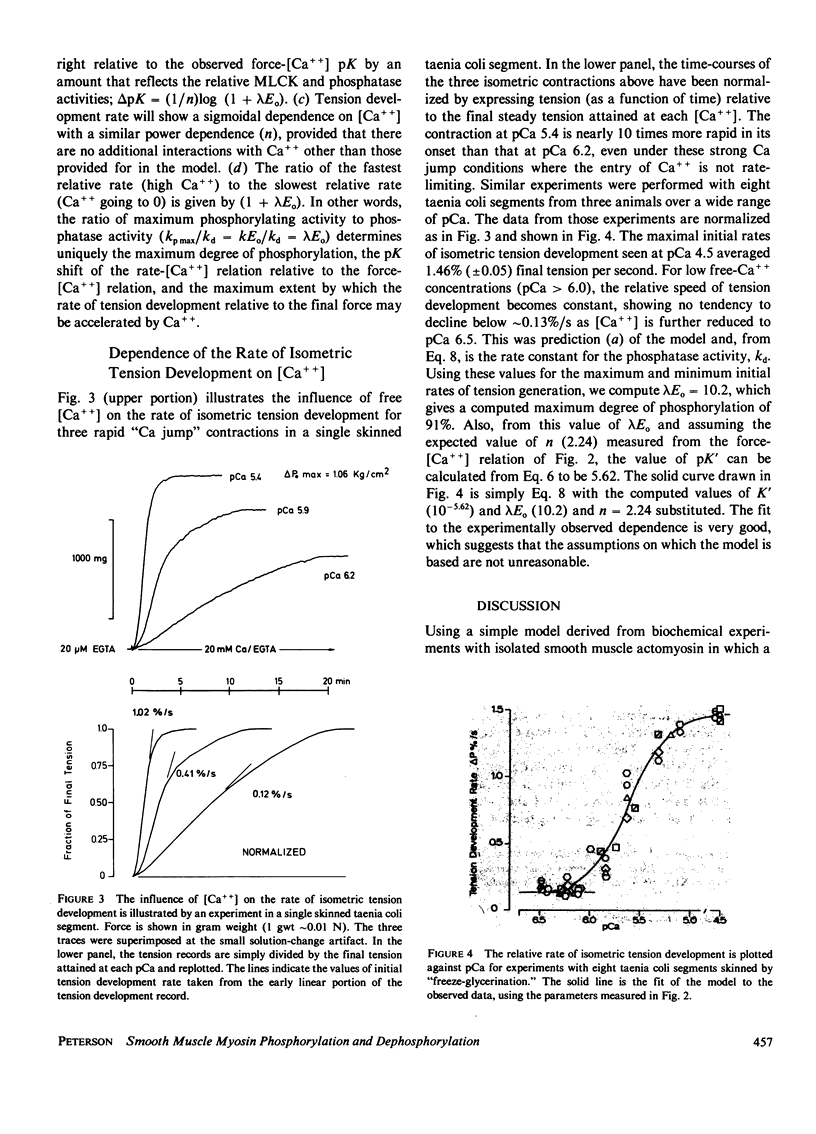
Abstract
A simple mathematical treatment of the model proposed by others in which a dynamic balance between Ca++ -dependent phosphorylation and Ca++-independent dephosphorylation of myosin controls the activation of smooth muscle contractility is presented. The parameters of the model can be computed from the experimentally observed stable force-[Ca++] relationship. A simple extension of the model to the case of time-dependent activation yields an expression that quantitatively predicts the measured dependence of the rate of isometric tension development on the activating free [Ca++]. The parameters of the mechanical model, which are derived from the rate constants for phosphorylating and dephosphorylating enzyme activities, are in reasonable agreement with the constants measured directly in purified protein systems. In addition, the model predicts values for several parameters that have not yet been experimentally measured, such as the ratio of kinase and phosphatase activities, the maximum extent of myosin phosphorylation, and the kinase turnover number.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy M. O., Williams D., Sharkey E. M., Hartshorne D. J. A relationship between Ca2+ sensitivity and phosphorylation of gizzard actomyosin. Biochem Biophys Res Commun. 1976 Mar 8;69(1):35–41. doi: 10.1016/s0006-291x(76)80268-9. [DOI] [PubMed] [Google Scholar]
- Barron J. T., Bárány M., Bárány K. Phosphorylation of the 20,000-dalton light chain of myosin of intact arterial smooth muscle in rest and in contraction. J Biol Chem. 1979 Jun 25;254(12):4954–4956. [PubMed] [Google Scholar]
- Chacko S. Effects of phosphorylation, calcium ion, and tropomyosin on actin-activated adenosine 5'-triphosphatase activity of mammalian smooth muscle myosin. Biochemistry. 1981 Feb 17;20(4):702–707. doi: 10.1021/bi00507a005. [DOI] [PubMed] [Google Scholar]
- Cohen D. M., Murphy R. A. Differences in cellular contractile protein contents among porcine smooth muscles: evidence for variation in the contractile system. J Gen Physiol. 1978 Sep;72(3):369–380. doi: 10.1085/jgp.72.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSalvo J., Gruenstein E., Silver P. Ca2+ dependent phosphorylation of bovine aortic actomyosin. Proc Soc Exp Biol Med. 1978 Jul;158(3):410–414. doi: 10.3181/00379727-158-40215. [DOI] [PubMed] [Google Scholar]
- Driska S. P., Aksoy M. O., Murphy R. A. Myosin light chain phosphorylation associated with contraction in arterial smooth muscle. Am J Physiol. 1981 May;240(5):C222–C233. doi: 10.1152/ajpcell.1981.240.5.C222. [DOI] [PubMed] [Google Scholar]
- Gordon A. R. Contraction of detergent-treated smooth muscle. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3527–3530. doi: 10.1073/pnas.75.7.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Kerrick W. G., Cassidy P. S. Chicken gizzard: relation between calcium-activated phosphorylation and contraction. Science. 1979 May 4;204(4392):503–506. doi: 10.1126/science.432654. [DOI] [PubMed] [Google Scholar]
- Kerrick W. G., Hoar P. E., Cassidy P. S. Calcium-activated tension: the role of myosin light chain phosphorylation. Fed Proc. 1980 Apr;39(5):1558–1563. [PubMed] [Google Scholar]
- Moisescu D. G. Kinetics of reaction in calcium-activated skinned muscle fibres. Nature. 1976 Aug 12;262(5569):610–613. doi: 10.1038/262610a0. [DOI] [PubMed] [Google Scholar]
- Mrwa U., Hartshorne D. J. Phosphorylation of smooth muscle myosin and myosin light chains. Fed Proc. 1980 Apr;39(5):1564–1568. [PubMed] [Google Scholar]
- Mrwa U., Troschka M., Gross C., Katzinski L. Calcium-sensitivity of pig-carotid-actomyosin ATPase in relation to phosphorylation of the regulatory light chain. Eur J Biochem. 1980 Jan;103(2):415–419. doi: 10.1111/j.1432-1033.1980.tb04328.x. [DOI] [PubMed] [Google Scholar]
- Peterson J. W. Vanadate ion inhibits actomyosin interaction in chemically skinned vascular smooth muscle. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1846–1853. doi: 10.1016/s0006-291x(80)80114-8. [DOI] [PubMed] [Google Scholar]
- Robertson S. P., Johnson J. D., Potter J. D. The time-course of Ca2+ exchange with calmodulin, troponin, parvalbumin, and myosin in response to transient increases in Ca2+. Biophys J. 1981 Jun;34(3):559–569. doi: 10.1016/S0006-3495(81)84868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry J. M., Górecka A., Aksoy M. O., Dabrowska R., Hartshorne D. J. Roles of calcium and phosphorylation in the regulation of the activity of gizzard myosin. Biochemistry. 1978 Oct 17;17(21):4411–4418. doi: 10.1021/bi00614a009. [DOI] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]


