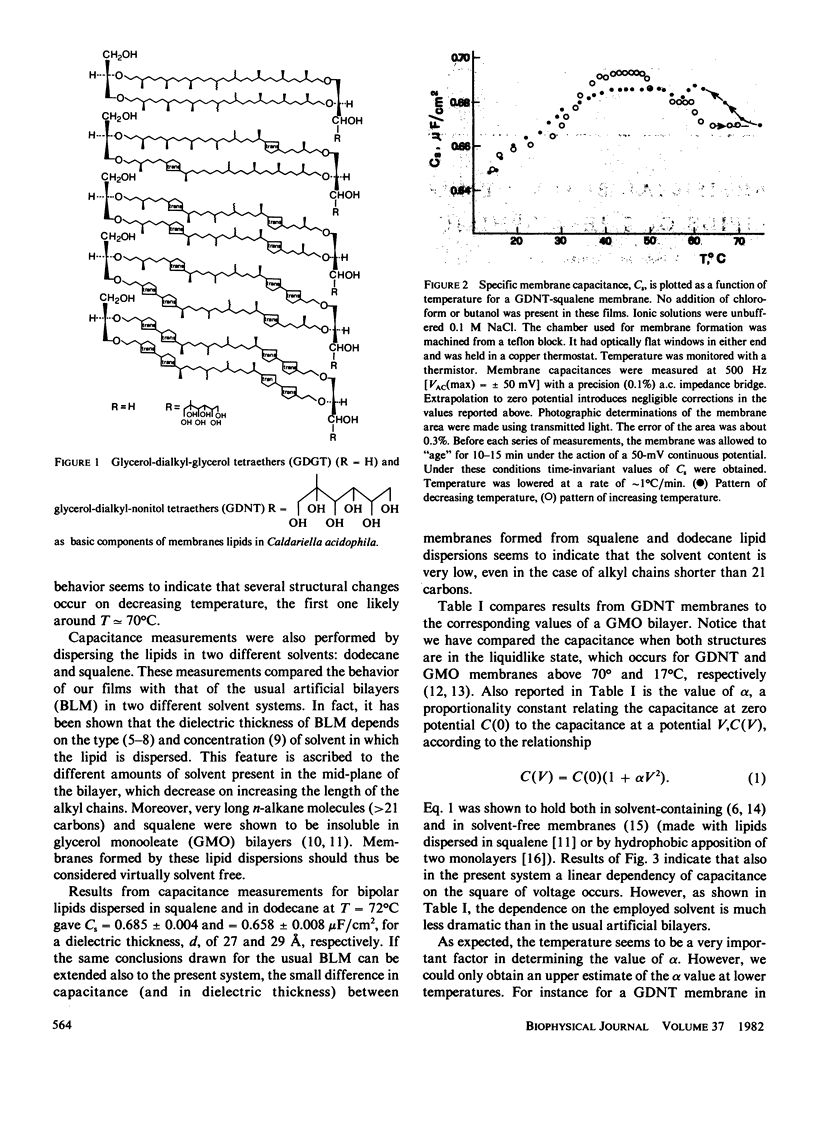
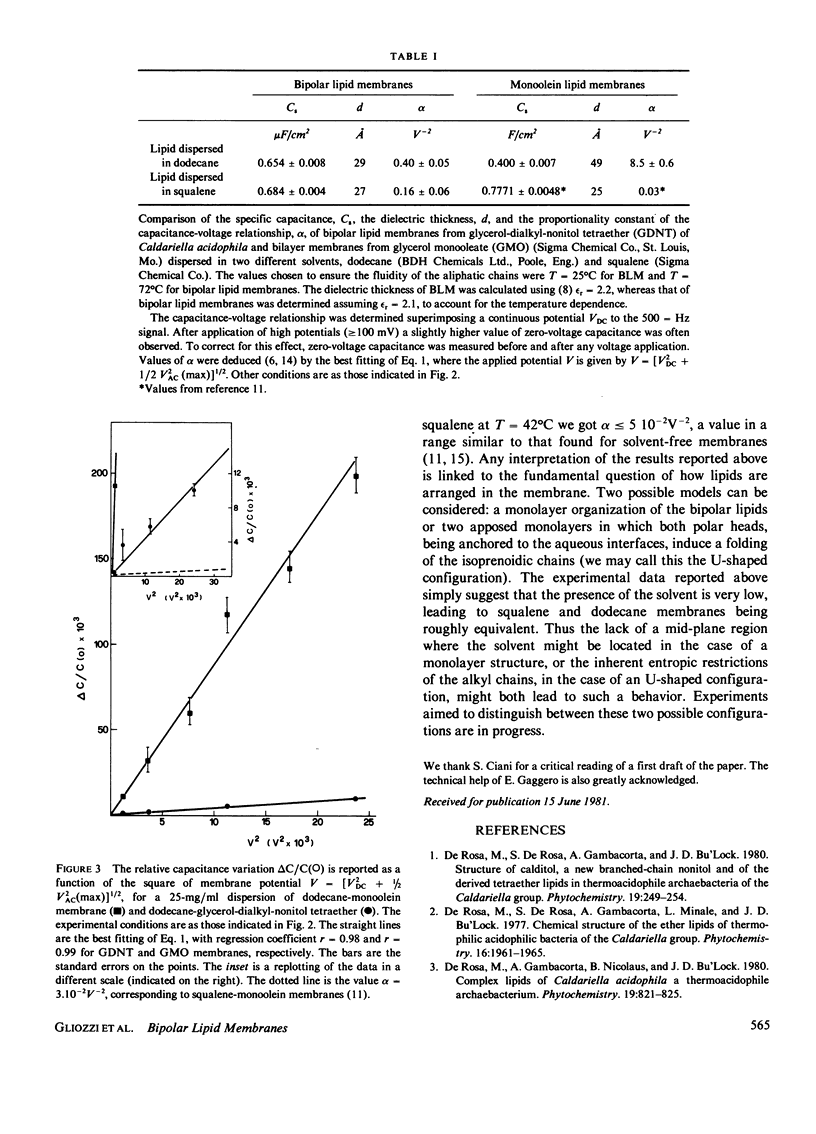
Abstract
The membrane of thermophilic archaebacteria is characterized by the presence of unusual isoprenoid bipolar lipids. The molecular organization of these lipids is still a matter of study. Important information could come from forming artificial black membranes. Black films can be formed from n-alkane or squalene dispersions of bipolar lipids extracted from the membrane of Caldariella acidophila. Membrane formation occurred only above a critical temperature (approximately 70 degrees C) corresponding to the physiological one. At lower temperatures, special solvent systems (n-alkanes or squalene, butanol and n-alkanes or squalene, butanol chloroform) were required. To characterize the physical parameters of these membranes, conductance and capacitance measurements were performed. Conductance was in the range of 10(-8) - 10(-7) omega -1 cm -2 , where specific capacitance at T = 72 degrees C was Cs = 0.685 +/- 0.004 microF/cm2 and Cs = 0.658 +/- 0.08 microF/cm2, corresponding to a dielectric thickness of 27 and 29 A for squalene and dodecane dispersions, respectively. Capacitance was shown to vary as the square of membrane potential, as usual in lipid bilayers. Values of the proportionality constant alpha have been compared to those of solvent-containing and solvent-free bilayers. The behavior of capacitance as a function of temperature is also shown by lowering temperature; the occurrence of complex structural changes was indicated. All the experimental data suggest that the presence of solvent is very low. Two possible molecular configurations of the films are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez O., Latorre R. Voltage-dependent capacitance in lipid bilayers made from monolayers. Biophys J. 1978 Jan;21(1):1–17. doi: 10.1016/S0006-3495(78)85505-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Fröhlich O., Läuger P., Montal M. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta. 1975 Jul 3;394(3):323–334. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Cherry R. J., Chapman D. Phase transitions and heterogeneity in lipid bilayers. Science. 1973 Aug 10;181(4099):557–559. doi: 10.1126/science.181.4099.557. [DOI] [PubMed] [Google Scholar]
- Waldbillig R., Szabo C. Solvent-depleted bilayer membrane from concentrated lipid solutions. Nature. 1978 Apr 27;272(5656):839–840. doi: 10.1038/272839a0. [DOI] [PubMed] [Google Scholar]
- White S. H. Formation of "solvent-free" black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J. 1978 Sep;23(3):337–347. doi: 10.1016/S0006-3495(78)85453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Phase transitions in planar bilayer membranes. Biophys J. 1975 Feb;15(2 Pt 1):95–117. doi: 10.1016/s0006-3495(75)85795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Studies of the physical chemistry of planar bilayer membranes using high-precision measurements of specific capacitance. Ann N Y Acad Sci. 1977 Dec 30;303:243–265. [PubMed] [Google Scholar]
- White S. H., Thompson T. E. Capacitance, area, and thickness variations in thin lipid films. Biochim Biophys Acta. 1973 Sep 27;323(1):7–22. doi: 10.1016/0005-2736(73)90428-8. [DOI] [PubMed] [Google Scholar]


