Abstract
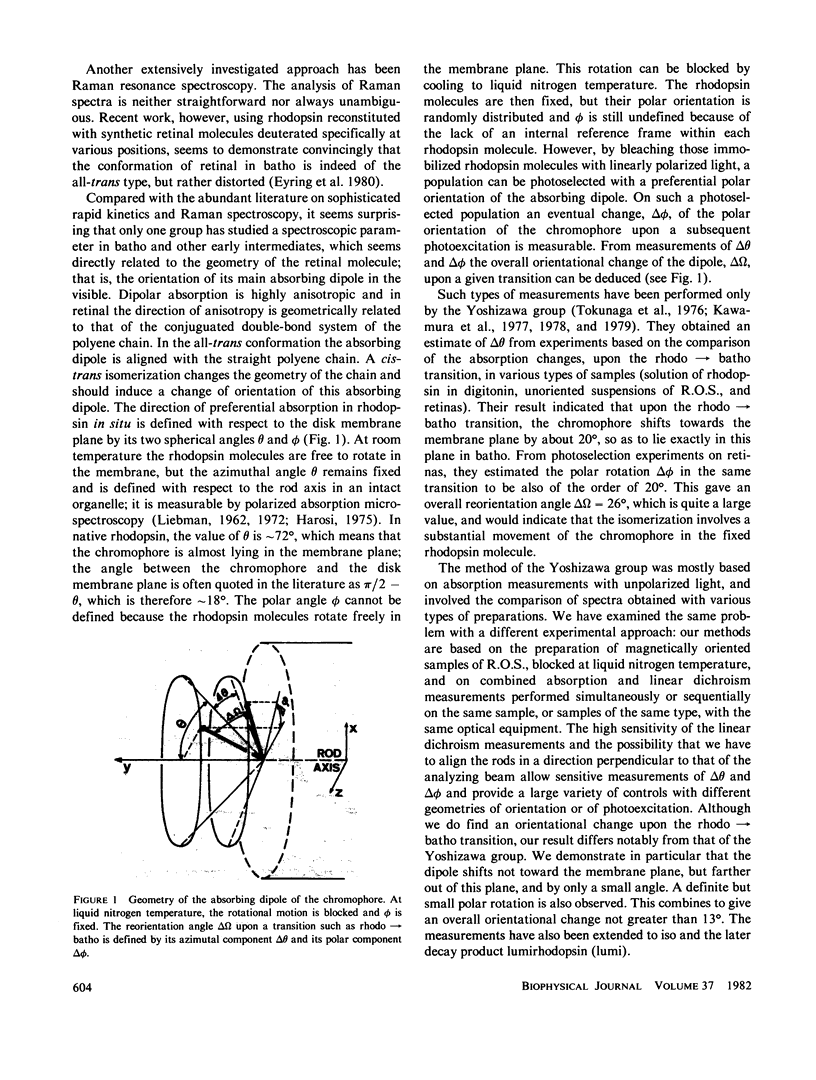
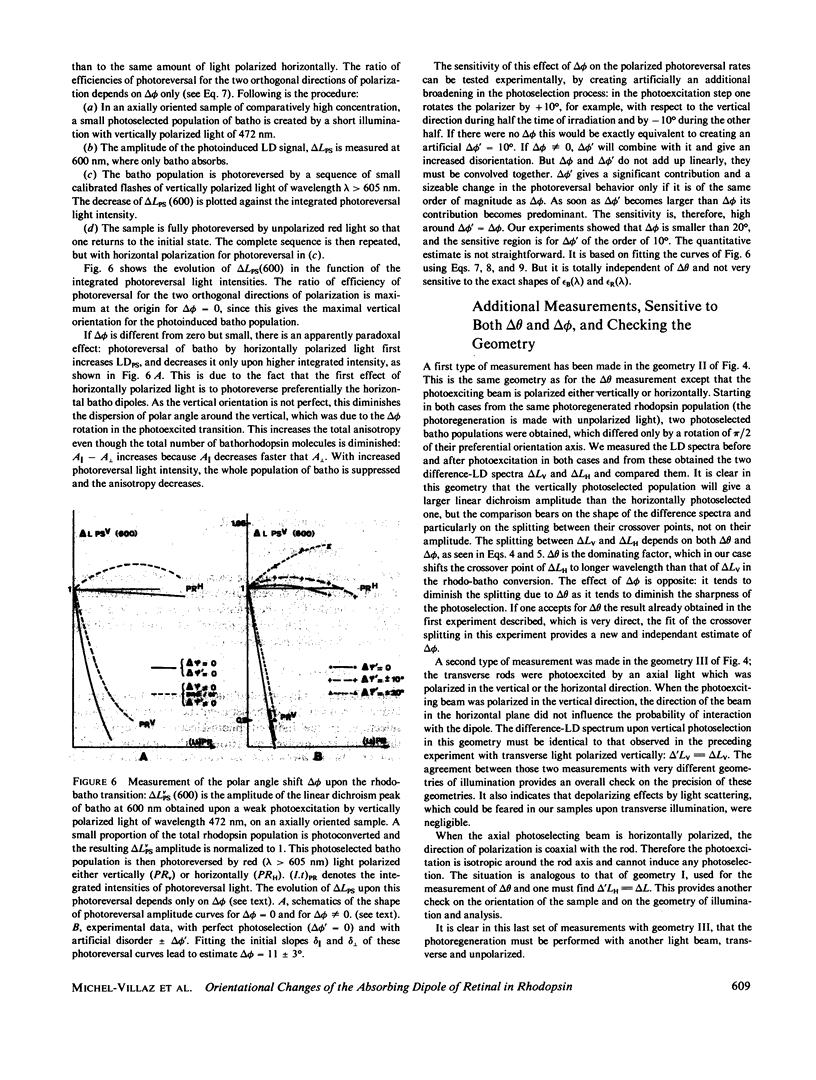
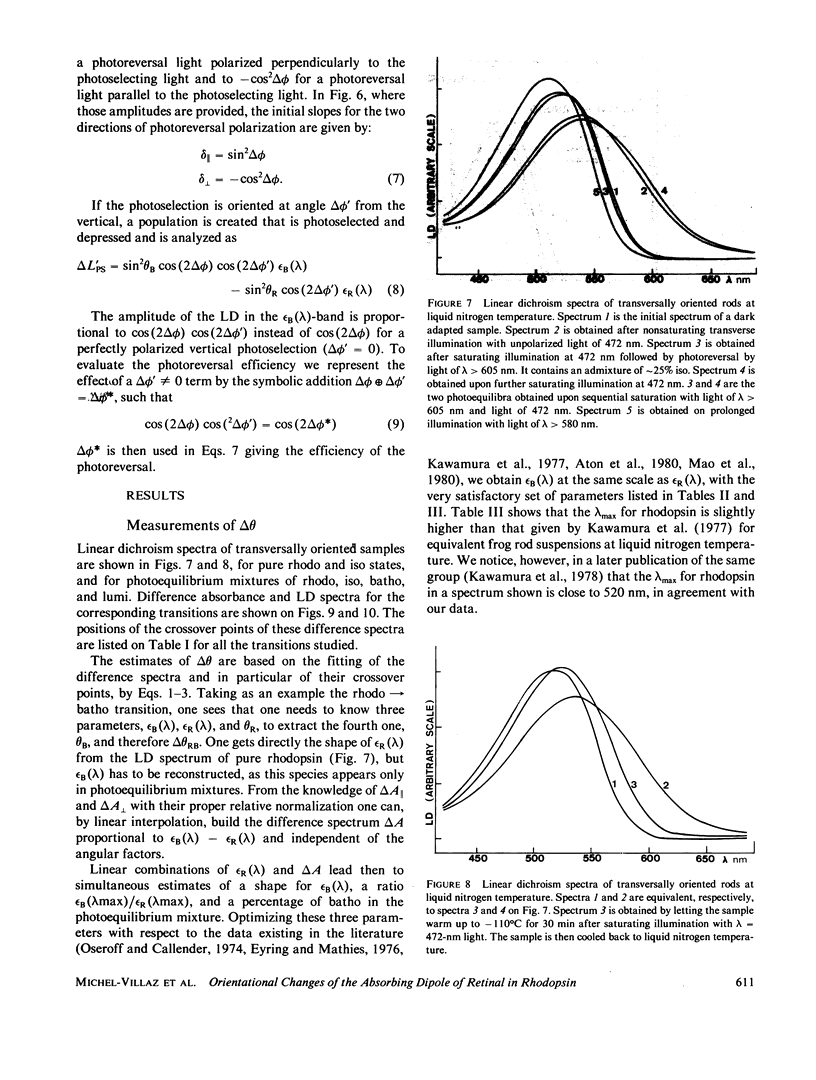
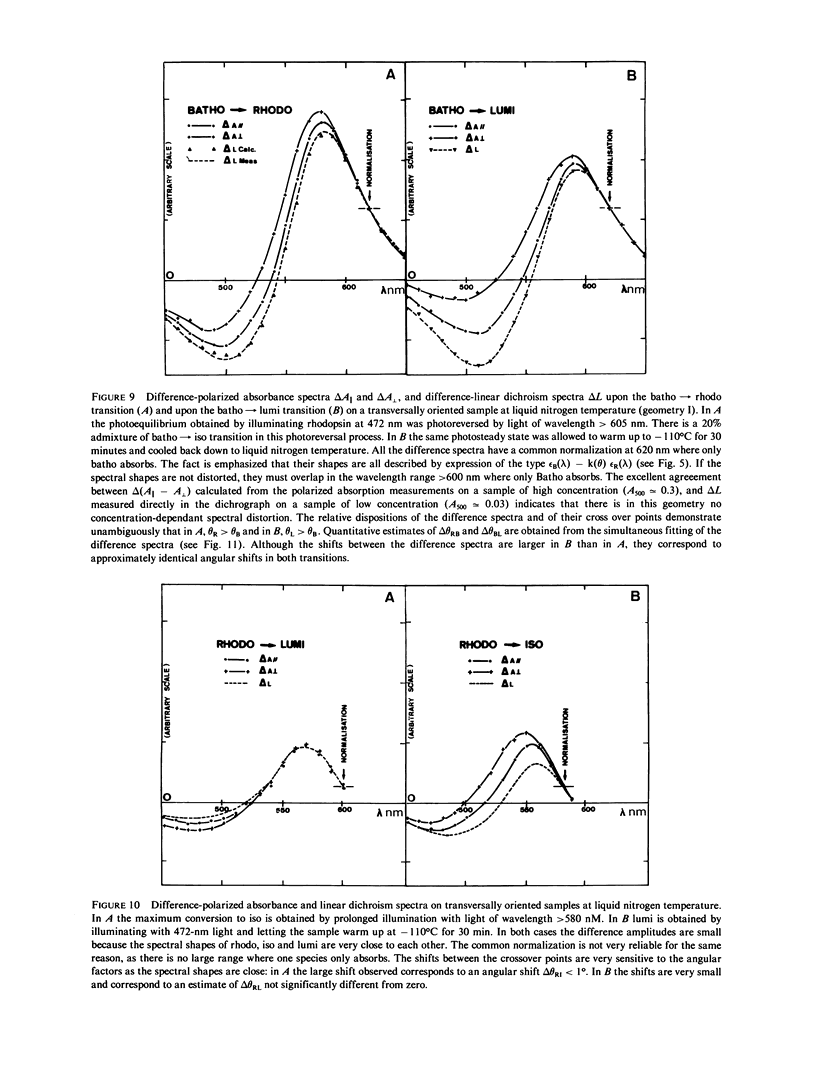
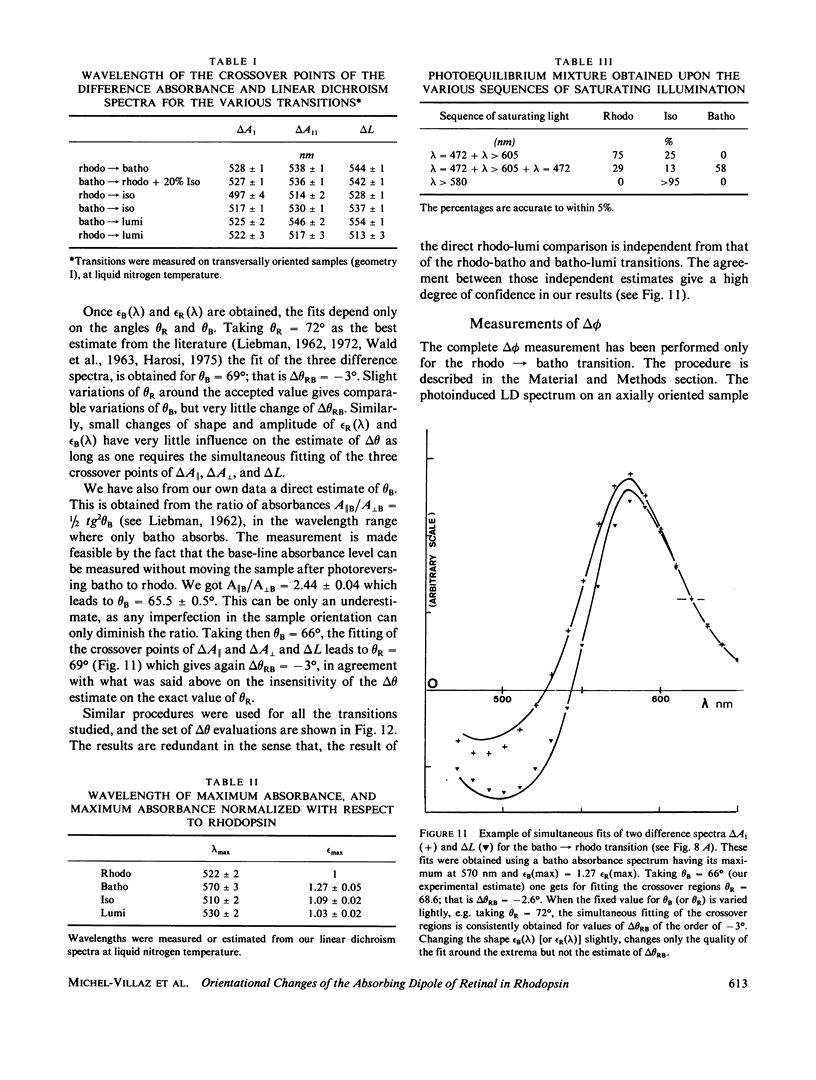
The orientational change of the absorbing dipole of the retinal chromophore in vertebrate rhodopsin (rhodo) upon photo-excitation to bathorhodopsin (batho), lumirhodopsin (lumi) and isorhodopsin (iso), has been studied by polarized absorption and linear dichroism measurements on magnetically oriented frog rod suspensions that were blocked at liquid nitrogen temperature. Both the azimuthal component delta theta and the polar component delta theta of the total angular change were studied in separate experiments. Delta theta was estimated from polarized absorption measurements on rods oriented transversally with respect to the analyzing beam. The data show unequivocally that upon the rhodo leads to batho transition, the dipole shifts out of the membrane plane by only few degrees; delta theta congruent to -3 degree. This azimuthal shift was nearly exactly reversed upon the batho leads to lumi decay. A very small shift (delta theta less than or equal to 1 degree) toward the membrane plane was observed upon a rhodo leads to iso conversion. The polar component delta theta of the angular shift was estimated by studying the photoreversion of linear dichroism induced by photo-excitation with polarized light in rods oriented parallel to the analyzing beam. Upon the rhodo leads to batho transition, ther was a shift delta theta = 11 +/- 3 degrees. The overall angular shift upon this first photo-exciting step, which corresponded to the isomerisation of retinal, was only delta omega = 11 +/- 3 degrees. This is smaller than what may be expected for a cis-trans isomerization of a retinal molecule with one end fixed, and different from what has been previously estimated by another group. These discrepancies are discussed.
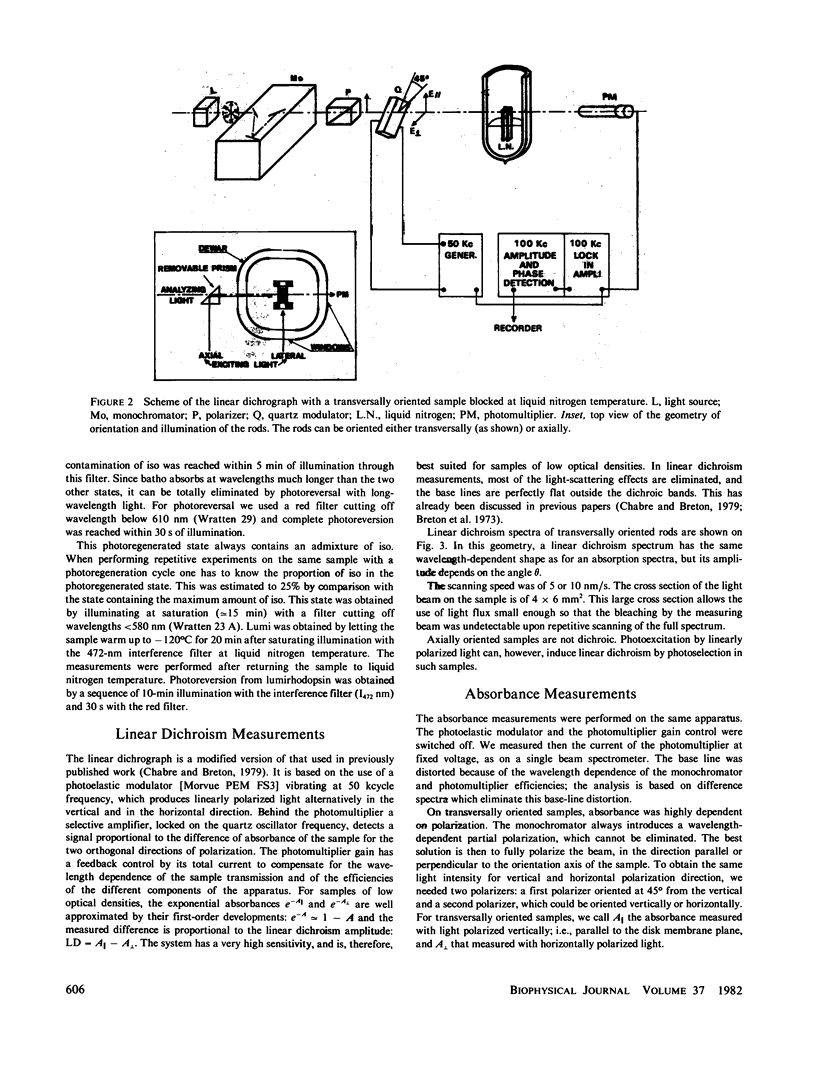
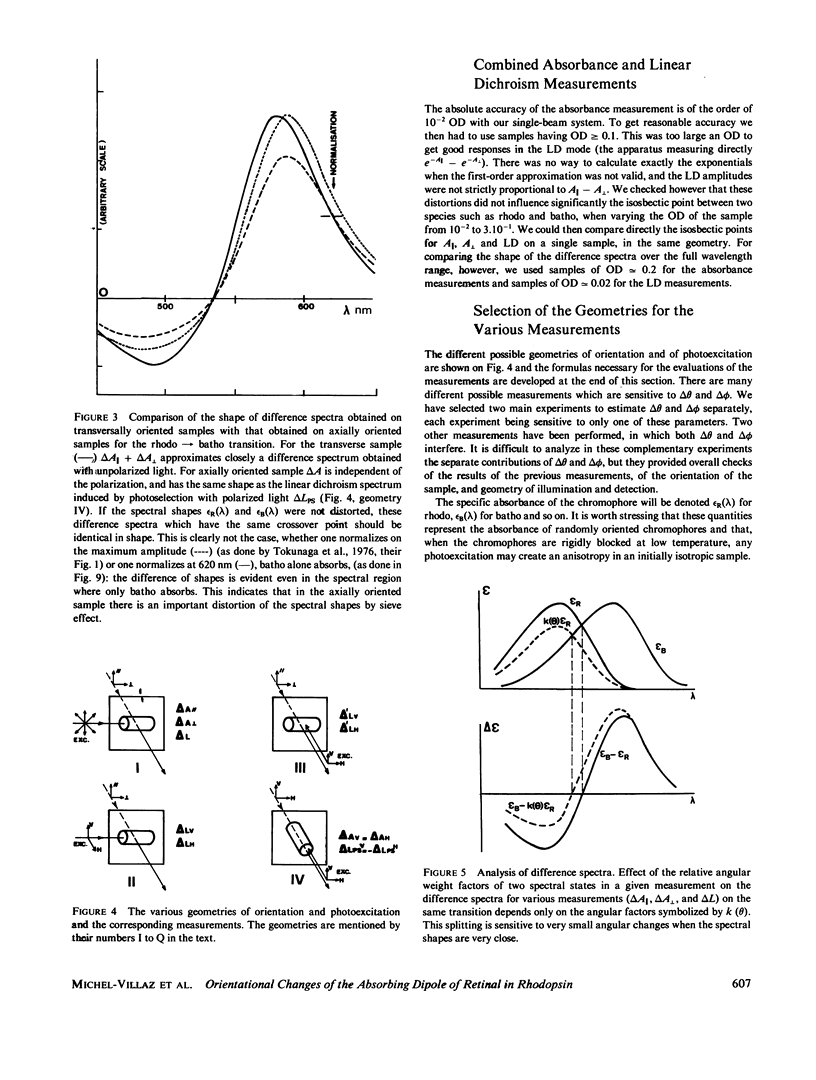
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aton B., Doukas A. G., Narva D., Callender R. H., Dinur U., Honig B. Resonance Raman studies of the primary photochemical event in visual pigments. Biophys J. 1980 Jan;29(1):79–94. doi: 10.1016/S0006-3495(80)85119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton J., Michel-Villaz M., Paillotin G. Orientation of pigments and structural proteins in the photosynthetic membrane of spinach chloroplasts: a linear dichroism study. Biochim Biophys Acta. 1973 Jul 26;314(1):42–56. doi: 10.1016/0005-2728(73)90062-5. [DOI] [PubMed] [Google Scholar]
- Chabre M., Breton J. The orientation of the chromophore of vertebrate rhodopsin in the "meta" intermediate states and the reversibility of the meta II-meta III transition. Vision Res. 1979;19(9):1005–1018. doi: 10.1016/0042-6989(79)90226-8. [DOI] [PubMed] [Google Scholar]
- Chabre M. X-ray diffraction studies of retinal rods. I. Structure of the disc membrane, effect of illumination. Biochim Biophys Acta. 1975 Mar 25;382(3):322–335. doi: 10.1016/0005-2736(75)90274-6. [DOI] [PubMed] [Google Scholar]
- Cooper A. Energy uptake in the first step of visual excitation. Nature. 1979 Nov 29;282(5738):531–533. doi: 10.1038/282531a0. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Eyring G., Curry B., Mathies R., Fransen R., Palings I., Lugtenburg J. Interpretation of the resonance Raman spectrum of bathorhodopsin based on visual pigment analogues. Biochemistry. 1980 May 27;19(11):2410–2418. doi: 10.1021/bi00552a020. [DOI] [PubMed] [Google Scholar]
- Gordon D. J., Holzwarth G. Artifacts in the measured optic activity of membrane suspensions. Arch Biochem Biophys. 1971 Feb;142(2):481–488. doi: 10.1016/0003-9861(71)90511-x. [DOI] [PubMed] [Google Scholar]
- Green B. H., Monger T. G., Alfano R. R., Aton B., Callender R. H. Cis-trans isomerisation in rhodopsin occurs in picoseconds. Nature. 1977 Sep 8;269(5624):179–180. doi: 10.1038/269179a0. [DOI] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakitani T., Kakitani H. Molecular mechanism for the initial process of visual excitation. III. Theoretical studies of optical spectra and conformations of chromophores in visual pigments, their analogues and intermdiates based on the torsion model. Biophys Struct Mech. 1979 Mar 21;5(1):55–73. doi: 10.1007/BF00535773. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Tokunaga F., Yoshizawa T. Absorption spectra of rhodopsin and its intermediates and orientational change of the chromophore. Vision Res. 1977;17(9):991–999. doi: 10.1016/0042-6989(77)90001-3. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Tokunaga F., Yoshizawa T., Sarai A., Kakitani T. Orientational changes of the transition dipole moment of retinal chromophore on the disk membrane due to the conversion of rhodopsin to bathorhodopsin and to isorhodopsin. Vision Res. 1979;19(8):879–884. doi: 10.1016/0042-6989(79)90021-x. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Wakabayashi S., Maeda A., Yoshizawa T. Isorhodopsin: conformation and orientation of its chromophore in frog disk membrane. Vision Res. 1978;18(4):457–462. doi: 10.1016/0042-6989(78)90057-3. [DOI] [PubMed] [Google Scholar]
- LIEBMAN P. A. In situ microspectrophotometric studies on the pigments of single retinal rods. Biophys J. 1962 Mar;2:161–178. doi: 10.1016/s0006-3495(62)86847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B., Ebrey T. G., Crouch R. Bathoproducts of rhodopsin, isorhodopsin I, and isorhodopsin II. Biophys J. 1980 Feb;29(2):247–256. doi: 10.1016/S0006-3495(80)85129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseroff A. R., Callender R. H. Resonance Raman spectroscopy of rhodopsin in retinal disk membranes. Biochemistry. 1974 Sep 24;13(20):4243–4248. doi: 10.1021/bi00717a027. [DOI] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling W., Rafferty C. N. Relationship between absorption spectrum and molecular conformations of 11-cis-retinal. Nature. 1969 Nov 8;224(5219):590–594. doi: 10.1038/224591a0. [DOI] [PubMed] [Google Scholar]
- Tokunaga F., Kawamura S., Yoshizawa T. Analysis by spectral difference of the orientational change of the rhodopsin chromophore during bleaching. Vision Res. 1976;16(6):633–641. doi: 10.1016/0042-6989(76)90011-0. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K., GIBBONS I. R. The problem of visual excitation. J Opt Soc Am. 1963 Jan;53:20–35. doi: 10.1364/josa.53.000020. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]


