Abstract
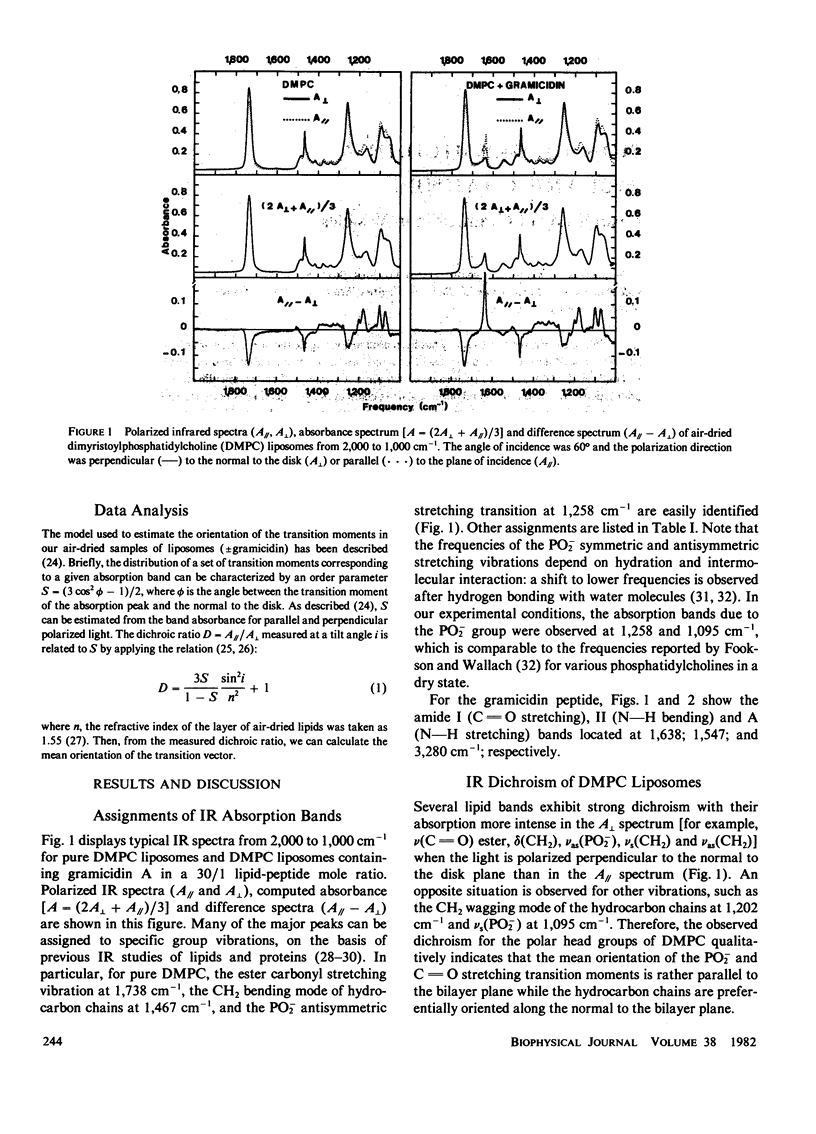
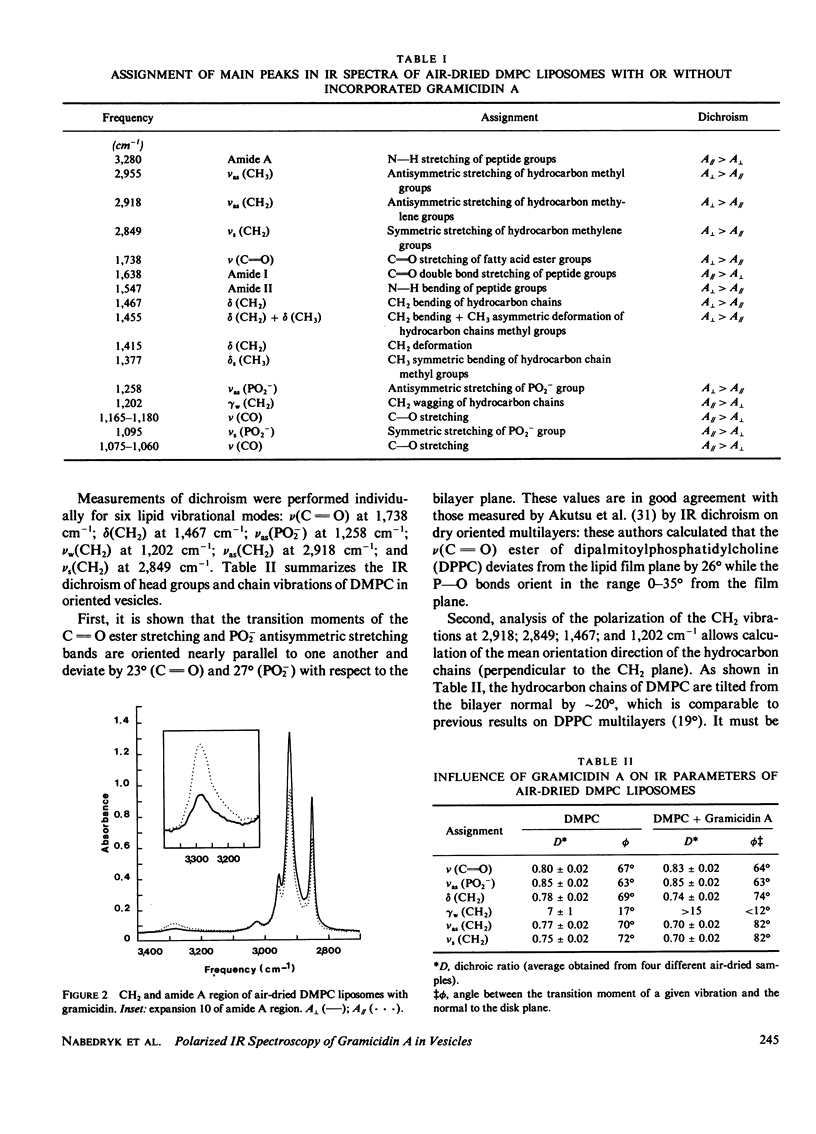
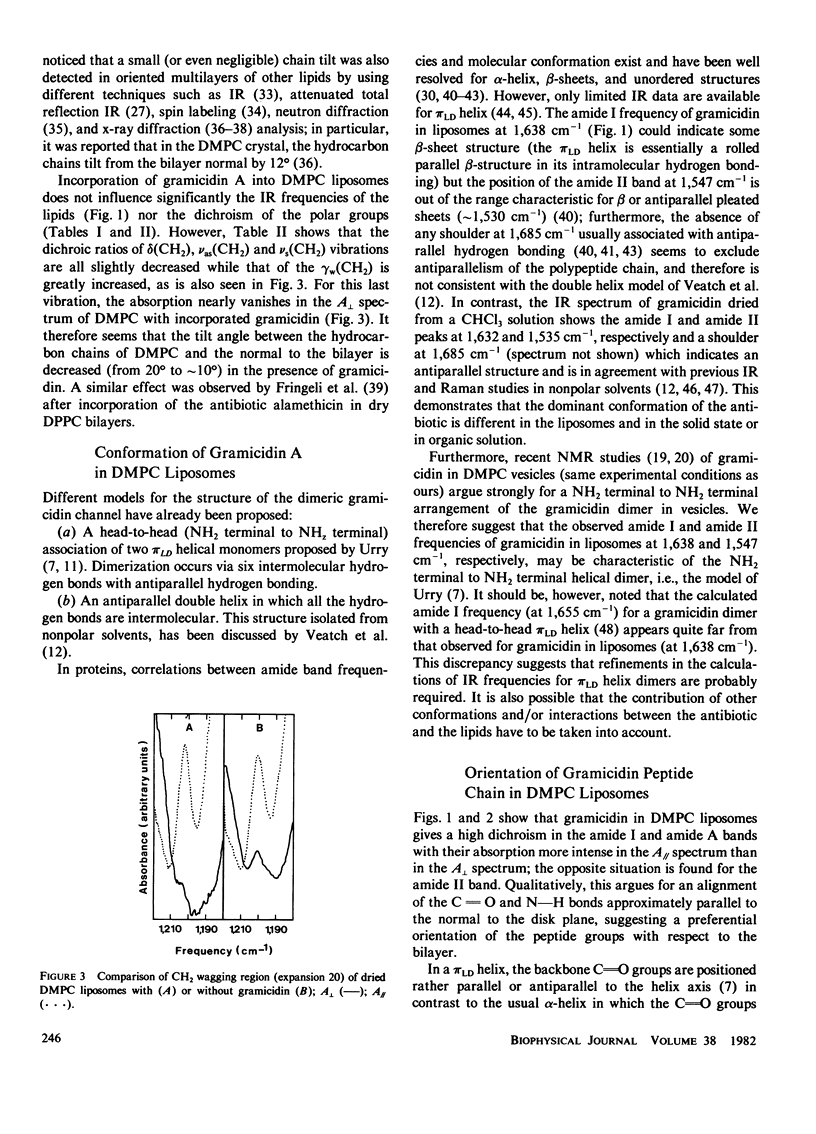
Polarized infrared spectroscopy has been used to investigate the orientation of gramicidin A incorporated in dimyristoylphosphatidylcholine liposomes. Dichroism measurements of the major lipid (C = O ester, PO2-, CH2) and peptide (amide A, I, II) bands were performed on liposomes (with or without gramicidin) oriented by air-drying. The mean orientation of the lipid groups and of the pi LD helix chain in the gramicidin has been determined. It can be inferred from infrared frequencies of gramicidin that the dominant conformation of the peptide in liposomes cannot be identified to the antiparallel double-helical dimer found in organic solution. No shift in lipid frequencies was observed upon incorporation of gramicidin in the liposomes. However, a slight reorganization of the lipid hydrocarbon chains which become oriented more closely to the normal to the bilayer is evidenced by a change in the dichroism of the CH2 vibrations. The infrared dichroism results of gramicidin imply a perpendicular orientation of the gramicidin transmembrane channel with the pi LD helix axis at less than 15 degrees with respect to the normal to the bilayer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akutsu H., Kyogoku Y. Conformational analysis of phosphatidylethanol-amine in multilayers by infrared dichroism. Chem Phys Lipids. 1975 Nov;15(2):222–242. doi: 10.1016/0009-3084(75)90045-6. [DOI] [PubMed] [Google Scholar]
- Apell H. J., Bamberg E., Alpes H., Läuger P. Formation of ion channels by a negatively charged analog of gramicidin A. J Membr Biol. 1977 Feb 24;31(1-2):171–188. doi: 10.1007/BF01869403. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Apell H. J., Alpes H. Structure of the gramicidin A channel: discrimination between the piL,D and the beta helix by electrical measurements with lipid bilayer membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2402–2406. doi: 10.1073/pnas.74.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Janko K. The action of a carbonsuboxide dimerized gramicidin A on lipid bilayer membranes. Biochim Biophys Acta. 1977 Mar 17;465(3):486–499. doi: 10.1016/0005-2736(77)90267-x. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Channel formation kinetics of gramicidin A in lipid bilayer membranes. J Membr Biol. 1973;11(2):177–194. doi: 10.1007/BF01869820. [DOI] [PubMed] [Google Scholar]
- Birrell G. B., Griffith O. H. Angle of tilt and domain structure in dipalmitoyl phosphatidylcholine multilayers. Arch Biochem Biophys. 1976 Feb;172(2):455–462. doi: 10.1016/0003-9861(76)90098-9. [DOI] [PubMed] [Google Scholar]
- Bradley R. J., Urry D. W., Okamoto K., Rapaka R. Channel structures of gramicidin: characterization of succinyl derivatives. Science. 1978 Apr 28;200(4340):435–437. doi: 10.1126/science.77040. [DOI] [PubMed] [Google Scholar]
- Breton J., Michel-Villaz M., Paillotin G. Orientation of pigments and structural proteins in the photosynthetic membrane of spinach chloroplasts: a linear dichroism study. Biochim Biophys Acta. 1973 Jul 26;314(1):42–56. doi: 10.1016/0005-2728(73)90062-5. [DOI] [PubMed] [Google Scholar]
- Büldt G., Gally H. U., Seelig A., Seelig J., Zaccai G. Neutron diffraction studies on selectively deuterated phospholipid bilayers. Nature. 1978 Jan 12;271(5641):182–184. doi: 10.1038/271182a0. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgadze Y. N., Nevskaya N. A. Infrared spectra and resonance interaction of amide-I vibration of the antiparallel-chain pleated sheet. Biopolymers. 1976 Apr;15(4):607–625. doi: 10.1002/bip.1976.360150402. [DOI] [PubMed] [Google Scholar]
- Eckert K., Grosse R., Malur J., Repke K. R. Calculation and use of protein-derived conformation-related spectra for the estimate of the secondary structure of proteins from their infrared spectra. Biopolymers. 1977 Nov;16(11):2549–2563. doi: 10.1002/bip.1977.360161116. [DOI] [PubMed] [Google Scholar]
- Fookson J. E., Wallach D. F. Structural differences among phosphatidylcholine, phosphatidylethanolamine, and mixed phosphatidylcholine/phosphatidylethanolamine multilayers: an infrared absorption study. Arch Biochem Biophys. 1978 Jul;189(1):195–204. doi: 10.1016/0003-9861(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Fringeli U. P., Fringeli M. Pore formation in lipid membranes by alamethicin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3852–3856. doi: 10.1073/pnas.76.8.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fringeli U. P. The structure of lipids and proteins studied by attenuated total reflection (ATR) infrared spectroscopy. II. Oriented layers of a homologous series: phosphatidylethanolamine to phosphatidylcholine. Z Naturforsch C. 1977 Jan-Feb;32(1-2):20–45. doi: 10.1515/znc-1977-1-205. [DOI] [PubMed] [Google Scholar]
- Goodall M. C. Structural effects in the action of antibiotics on the ion permeability of lipid bilayers. 3. Gramicidins "A" and "S", and lipid specificity. Biochim Biophys Acta. 1970 Dec 1;219(2):471–478. doi: 10.1016/0005-2736(70)90225-7. [DOI] [PubMed] [Google Scholar]
- Heitz F., Lotz B., Spach G. AlphaDL and piDL helices of alternating poly-gamma-benzyl-D-L-glutamate. J Mol Biol. 1975 Feb 15;92(1):1–13. doi: 10.1016/0022-2836(75)90088-1. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Discreteness of conductance change in bimolecular lipid membranes in the presence of certain antibiotics. Nature. 1970 Jan 31;225(5231):451–453. doi: 10.1038/225451a0. [DOI] [PubMed] [Google Scholar]
- Iqbal Z., Weidekamm E. Raman and infrared spectroscopic study of gramicidin A conformations. Arch Biochem Biophys. 1980 Jul;202(2):639–649. doi: 10.1016/0003-9861(80)90472-5. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Lavialle F., Levin I. W., Mollay C. Interaction of melittin with dimyristoyl phosphatidylcholine liposomes: evidence for boundary lipid by Raman spectroscopy. Biochim Biophys Acta. 1980 Jul 16;600(1):62–71. doi: 10.1016/0005-2736(80)90411-3. [DOI] [PubMed] [Google Scholar]
- Lotz B., Colonna-Cesari F., Heitz F., Spach G. A family of double helices of alternating poly(gamma-benzyl-D-L-glutamate), a stereochemical model for gramicidin A. J Mol Biol. 1976 Oct 5;106(4):915–942. doi: 10.1016/0022-2836(76)90343-0. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. Differences in hydrocarbon chain tilt between hydrated phosphatidylethanolamine and phosphatidylcholine bilayers. A molecular packing model. Biophys J. 1980 Feb;29(2):237–245. doi: 10.1016/S0006-3495(80)85128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel-Villaz M., Saibil H. R., Chabre M. Orientation of rhodopsin alpha-helices in in retinal rod outer segment membranes studied by infrared linear dichroism. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4405–4408. doi: 10.1073/pnas.76.9.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S., Miyazaki T. Dichroism of bacteriochlorophyll in cells of the photosynthetic bacterium Rhodopseudomonas palustris. Biochim Biophys Acta. 1971 Aug 6;245(1):151–159. doi: 10.1016/0005-2728(71)90017-x. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Veatch W. R., Stryer L. Transmembrane channel activity of gramicidin A analogs: effects of modification and deletion of the amino-terminal residue. J Mol Biol. 1979 Aug 25;132(4):733–738. doi: 10.1016/0022-2836(79)90386-3. [DOI] [PubMed] [Google Scholar]
- Nabedryk E., Breton J. Orientation of intrinsic proteins in photosynthetic membranes. Polarized infrared spectroscopy of chloroplasts and chromatophores. Biochim Biophys Acta. 1981 May 13;635(3):515–524. doi: 10.1016/0005-2728(81)90110-9. [DOI] [PubMed] [Google Scholar]
- Nevskaya N. A., Chirgadze Y. N. Infrared spectra and resonance interactions of amide-I and II vibration of alpha-helix. Biopolymers. 1976 Apr;15(4):637–648. doi: 10.1002/bip.1976.360150404. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Clark N. A. Polarized infrared spectroscopy of oriented purple membrane. Biophys J. 1979 Mar;25(3):473–487. doi: 10.1016/S0006-3495(79)85317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Sanches R., Hsiao T. L., Clark N. A. A spectroscopic study of rhodopsin alpha-helix orientation. Biophys J. 1980 Jul;31(1):53–64. doi: 10.1016/S0006-3495(80)85040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Stanley H. E. Raman spectroscopic investigation of gramicidin A' conformations. Science. 1974 Aug 16;185(4151):616–618. doi: 10.1126/science.185.4151.616. [DOI] [PubMed] [Google Scholar]
- SARGES R., WITKOP B. GRAMICIDIN A. V. THE STRUCTURE OF VALINE- AND ISOLEUCINE-GRAMICIDIN A. J Am Chem Soc. 1965 May 5;87:2011–2020. doi: 10.1021/ja01087a027. [DOI] [PubMed] [Google Scholar]
- Stamatoff J. B., Graddick W. F., Powers L., Moncton D. E. Direct observation of the hydrocarbon chain tilt angle in phospholipid bilayers. Biophys J. 1979 Feb;25(2 Pt 1):253–261. doi: 10.1016/s0006-3495(79)85289-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G., Urry D. W. N-acetyl gramicidin: single-channel properties and implications for channel structure. Science. 1979 Jan 5;203(4375):55–57. doi: 10.1126/science.83000. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Goodall M. C., Glickson J. D., Mayers D. F. The gramicidin A transmembrane channel: characteristics of head-to-head dimerized (L,D) helices. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1907–1911. doi: 10.1073/pnas.68.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch W. R., Fossel E. T., Blout E. R. The conformation of gramicidin A. Biochemistry. 1974 Dec 17;13(26):5249–5256. doi: 10.1021/bi00723a001. [DOI] [PubMed] [Google Scholar]
- Veatch W. R., Mathies R., Eisenberg M., Stryer L. Simultaneous fluorescence and conductance studies of planar bilayer membranes containing a highly active and fluorescent analog of gramicidin A. J Mol Biol. 1975 Nov 25;99(1):75–92. doi: 10.1016/s0022-2836(75)80160-4. [DOI] [PubMed] [Google Scholar]
- Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J Mol Biol. 1977 Jun 15;113(1):89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F. Effect of melittin on thermotropic lipid state transitions in phosphatidylcholine liposomes. Biochim Biophys Acta. 1976 Apr 5;426(4):616–623. doi: 10.1016/0005-2736(76)90125-5. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F., Smith I. C. The action of melittin on phosphatide multibilayers as studied by infrared dichroism and spin labeling. A model approach to lipid-protein interactions. Biochim Biophys Acta. 1974 Apr 12;345(1):129–140. doi: 10.1016/0005-2736(74)90252-1. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Verma S. P., Fookson J. Application of laser Raman and infrared spectroscopy to the analysis of membrane structure. Biochim Biophys Acta. 1979 Aug 20;559(2-3):153–208. doi: 10.1016/0304-4157(79)90001-7. [DOI] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Morrow J. S., Veatch W. R. Conformation of the gramicidin A transmembrane channel: A 13C nuclear magnetic resonance study of 13C-enriched gramicidin in phosphatidylcholine vesicles. J Mol Biol. 1980 Oct 15;143(1):1–19. doi: 10.1016/0022-2836(80)90121-7. [DOI] [PubMed] [Google Scholar]


