Abstract
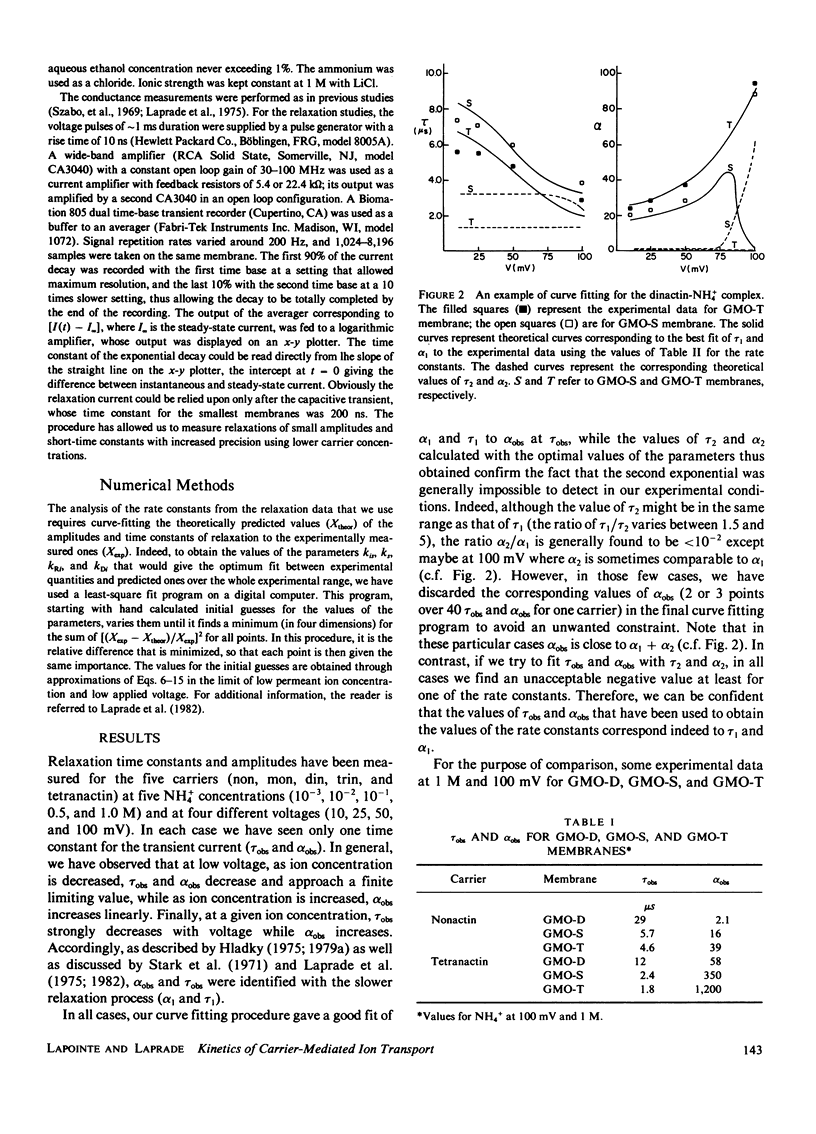
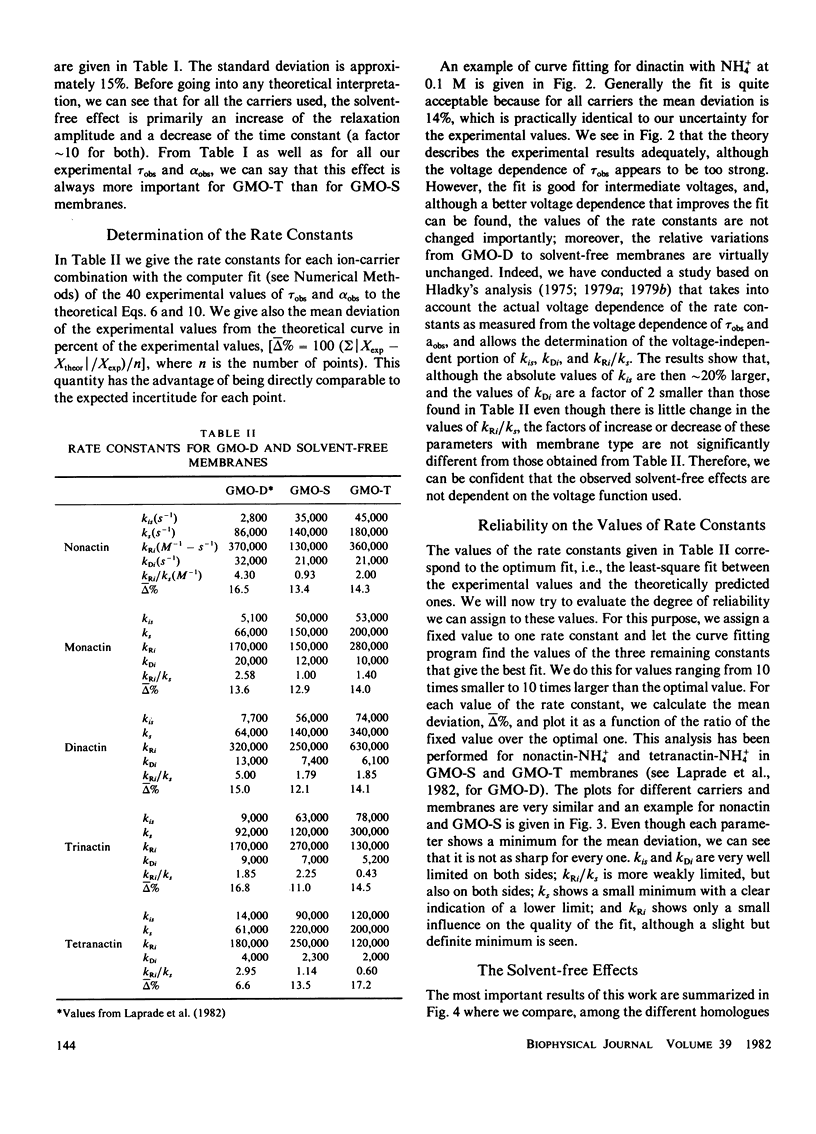
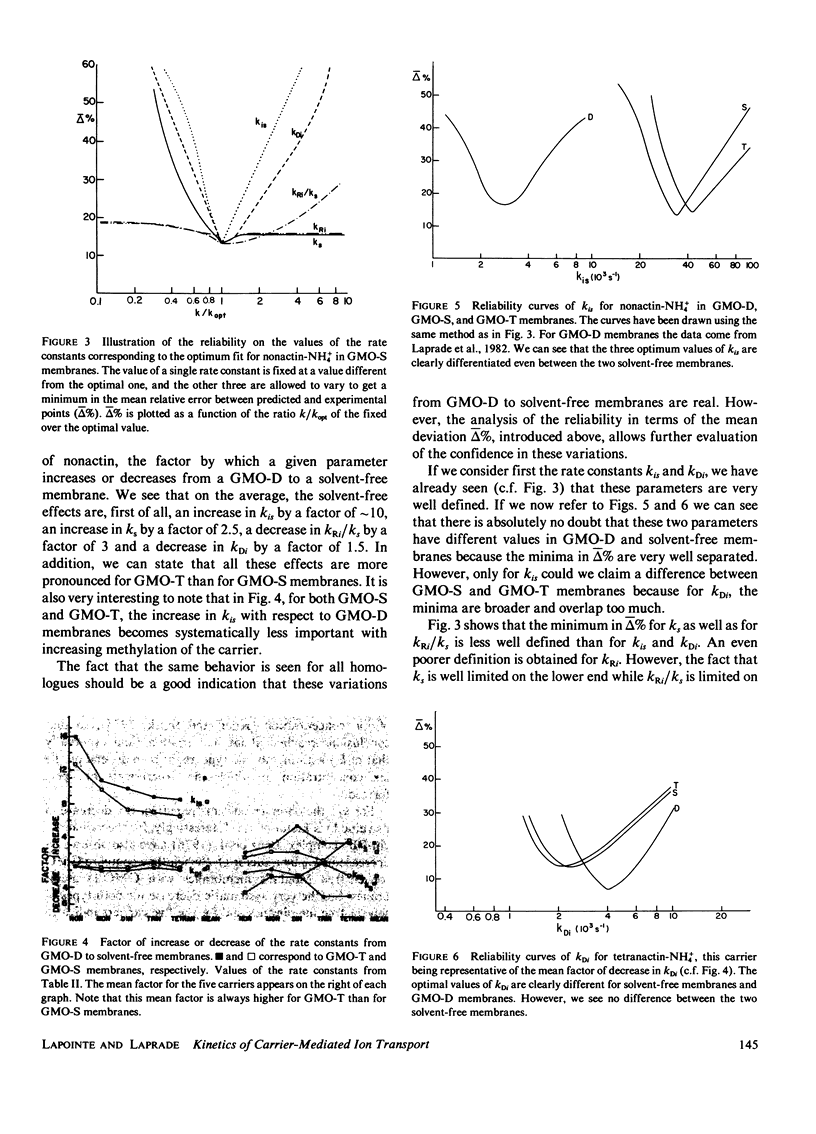
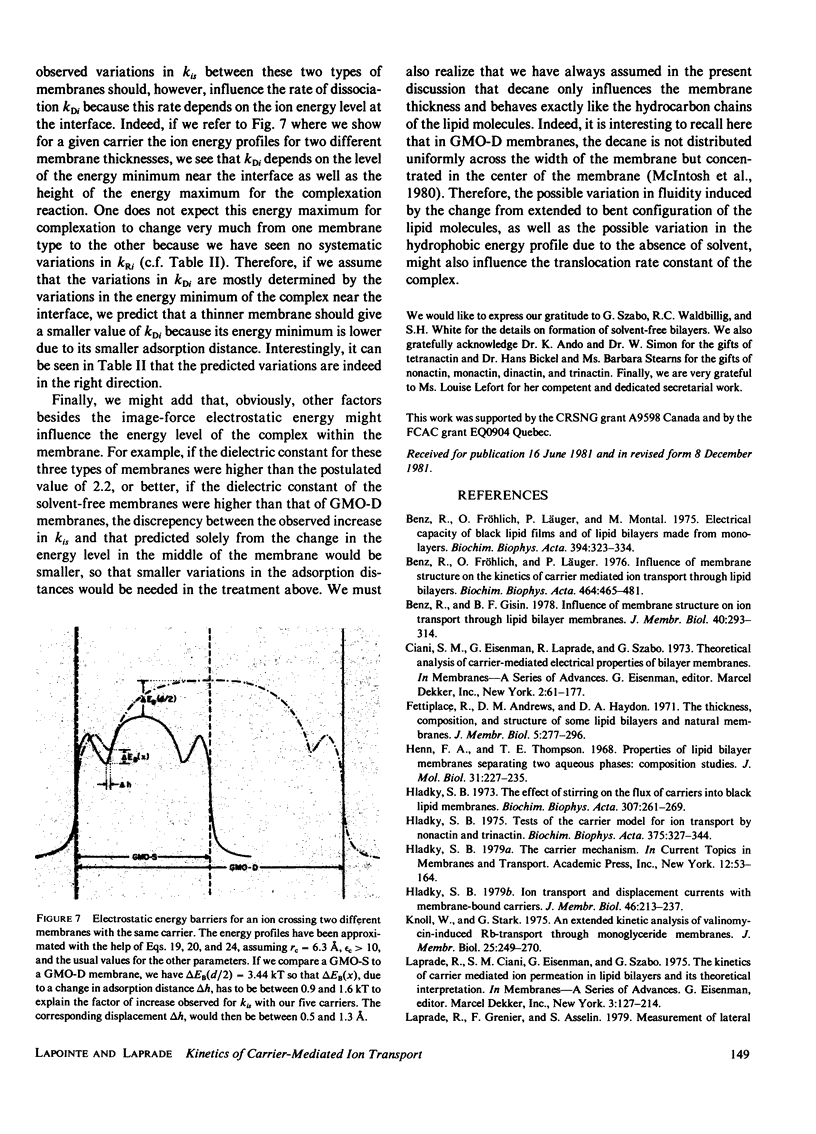
In contrast with the usual glyceryl-monooleate/decane (GMO-D) bilayer lipid membranes, new membranes, formed from a mixture of GMO in squalene (GMO-S) or from a mixture of GMO in triolein (GMO-T), seem to be almost solvent free. Our results from voltage-jump relaxation studies, using these "solvent-free" membranes with the homologue carriers, nonactin, monactin, dinactin, trinactin, and tetranactin, are compared with the corresponding ones for GMO-D membranes. With all homologues, solvent-free membranes show an increase of the free carrier translocation rate, ks, by a factor of 2.5, a decrease in the dissociation rate constant of the complex, kDi, by a factor of 1.5 and no significant change in its formation rate constant, kRi. However, the principal effect of the absence of solvent in these membranes is an increase by a factor of approximately 10 of the translocation rate constant for moving the complex across the membrane, kis. This increase varies regularly from a factor of 7-15 with decreasing carrier size, and is always larger for GMO-T than for GMO-S membranes. These solvent-free effects are interpreted in terms of modifications of electrostatic and hydrophobic energy profiles in the membrane.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Fröhlich O., Läuger P. Influence of membrane structure on the kinetics of carrier-mediated ion transport through lipid bilayers. Biochim Biophys Acta. 1977 Feb 4;464(3):465–481. doi: 10.1016/0005-2736(77)90023-2. [DOI] [PubMed] [Google Scholar]
- Benz R., Fröhlich O., Läuger P., Montal M. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta. 1975 Jul 3;394(3):323–334. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- Benz R., Gisin B. F. Influence of membrane structure on ion transport through lipid bilayer membranes. J Membr Biol. 1978 Jun 9;40(4):293–314. doi: 10.1007/BF01874161. [DOI] [PubMed] [Google Scholar]
- Ciani S. M., Eisenman G., Laprade R., Szabo G. Theoretical analysis of carrier-mediated electrical properties of bilayer membranes. Membranes. 1973;2:61–177. [PubMed] [Google Scholar]
- Henn F. A., Thompson T. E. Properties of lipid bilayer membranes separating two aqueous phases: composition studies. J Mol Biol. 1968 Jan 28;31(2):227–235. doi: 10.1016/0022-2836(68)90441-5. [DOI] [PubMed] [Google Scholar]
- Hladky S. B. Ion transport and displacement currents with membrane-bound carriers: the theory for voltage-clamp currents, charge-pulse transients and admittance for symmetrical systems. J Membr Biol. 1979;46(3):213–237. doi: 10.1007/BF01868765. [DOI] [PubMed] [Google Scholar]
- Hladky S. B. Tests of the carrier model for ion transport by nonactin and trinactin. Biochim Biophys Acta. 1975 Feb 14;375(3):327–349. doi: 10.1016/0005-2736(75)90351-x. [DOI] [PubMed] [Google Scholar]
- Hladky S. B. The effect of stirring on the flux of carriers into black lipid membranes. Biochim Biophys Acta. 1973 May 11;307(2):261–269. doi: 10.1016/0005-2736(73)90093-x. [DOI] [PubMed] [Google Scholar]
- Knoll W., Stark G. An extended kinetic analysis of valinomycin-induced Rb-transport through monoglyceride membranes. J Membr Biol. 1975;25(3-4):249–270. doi: 10.1007/BF01868578. [DOI] [PubMed] [Google Scholar]
- Laprade R., Ciani S., Eisenman G., Szabo G. The kinetics of carrier-mediated ion permeation in lipid bilayers and its theoretical interpreatation. Membranes. 1975;3:127–214. [PubMed] [Google Scholar]
- Läuger P., Stark G. Kinetics of carrier-mediated ion transport across lipid bilayer membranes. Biochim Biophys Acta. 1970 Sep 15;211(3):458–466. doi: 10.1016/0005-2736(70)90251-8. [DOI] [PubMed] [Google Scholar]
- MUELLER P., RUDIN D. O., TIEN H. T., WESCOTT W. C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature. 1962 Jun 9;194:979–980. doi: 10.1038/194979a0. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., MacDonald R. C. The organization of n-alkanes in lipid bilayers. Biochim Biophys Acta. 1980 Apr 24;597(3):445–463. doi: 10.1016/0005-2736(80)90219-9. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Läuger P. Nonlinear electrical effects in lipid bilayer membranes. II. Integration of the generalized Nernst-Planck equations. Biophys J. 1969 Sep;9(9):1160–1170. doi: 10.1016/S0006-3495(69)86443-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano R. E., Ruysschaert J. M., Miller I. R. The molecular composition of some lipid bilayer membranes in aqueous solution. J Membr Biol. 1972;10(1):11–30. doi: 10.1007/BF01867845. [DOI] [PubMed] [Google Scholar]
- Parsegian A. Energy of an ion crossing a low dielectric membrane: solutions to four relevant electrostatic problems. Nature. 1969 Mar 1;221(5183):844–846. doi: 10.1038/221844a0. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A. Ion-membrane interactions as structural forces. Ann N Y Acad Sci. 1975 Dec 30;264:161–171. doi: 10.1111/j.1749-6632.1975.tb31481.x. [DOI] [PubMed] [Google Scholar]
- Simon W., Morf W. E. Alkali cation specificity of carrier antibiotics and their behavior in bulk membranes. Membranes. 1973;2:329–375. [PubMed] [Google Scholar]
- Stark G., Ketterer B., Benz R., Läuger P. The rate constants of valinomycin-mediated ion transport through thin lipid membranes. Biophys J. 1971 Dec;11(12):981–994. doi: 10.1016/S0006-3495(71)86272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbillig R. C., Szabo G. Planar bilayer membranes from pure lipids. Biochim Biophys Acta. 1979 Nov 2;557(2):295–305. doi: 10.1016/0005-2736(79)90328-6. [DOI] [PubMed] [Google Scholar]
- Waldbillig R., Szabo C. Solvent-depleted bilayer membrane from concentrated lipid solutions. Nature. 1978 Apr 27;272(5656):839–840. doi: 10.1038/272839a0. [DOI] [PubMed] [Google Scholar]
- White S. H. Formation of "solvent-free" black lipid bilayer membranes from glyceryl monooleate dispersed in squalene. Biophys J. 1978 Sep;23(3):337–347. doi: 10.1016/S0006-3495(78)85453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H., Petersen D. C., Simon S., Yafuso M. Formation of planar bilayer membranes from lipid monolayers. A critique. Biophys J. 1976 May;16(5):481–489. doi: 10.1016/S0006-3495(76)85703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. H. Studies of the physical chemistry of planar bilayer membranes using high-precision measurements of specific capacitance. Ann N Y Acad Sci. 1977 Dec 30;303:243–265. [PubMed] [Google Scholar]


