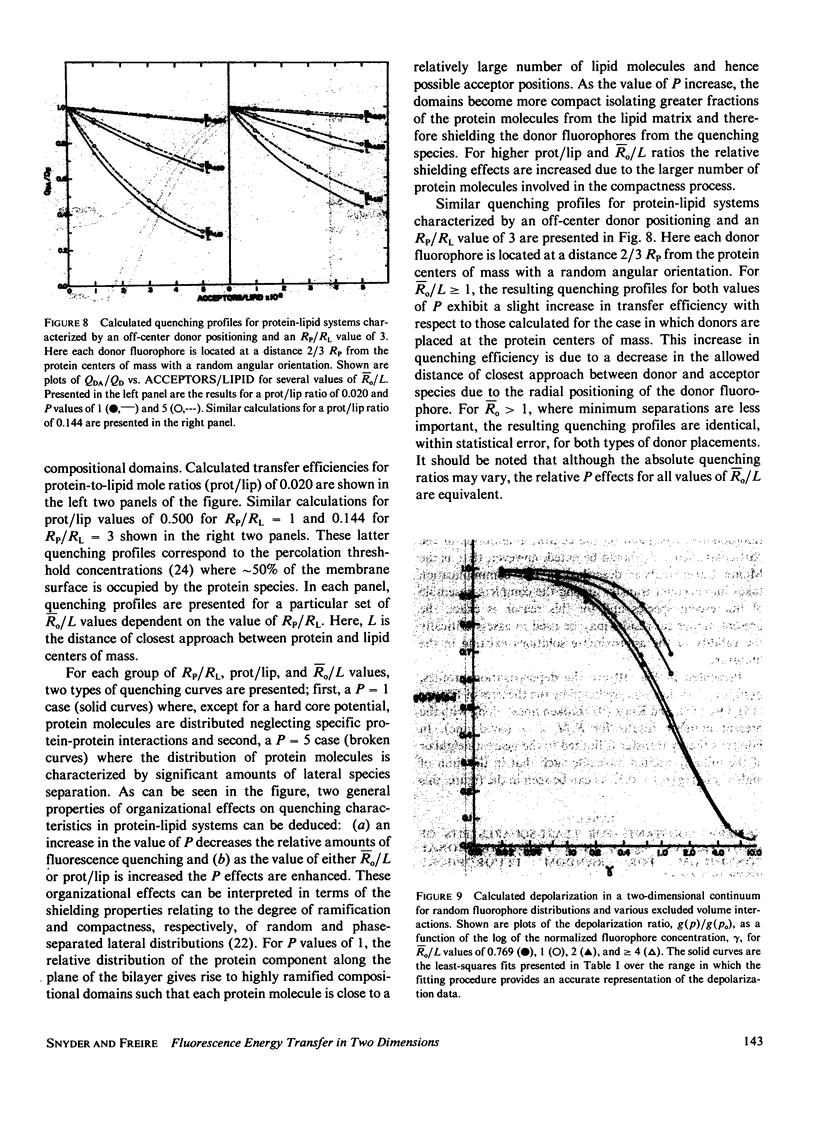
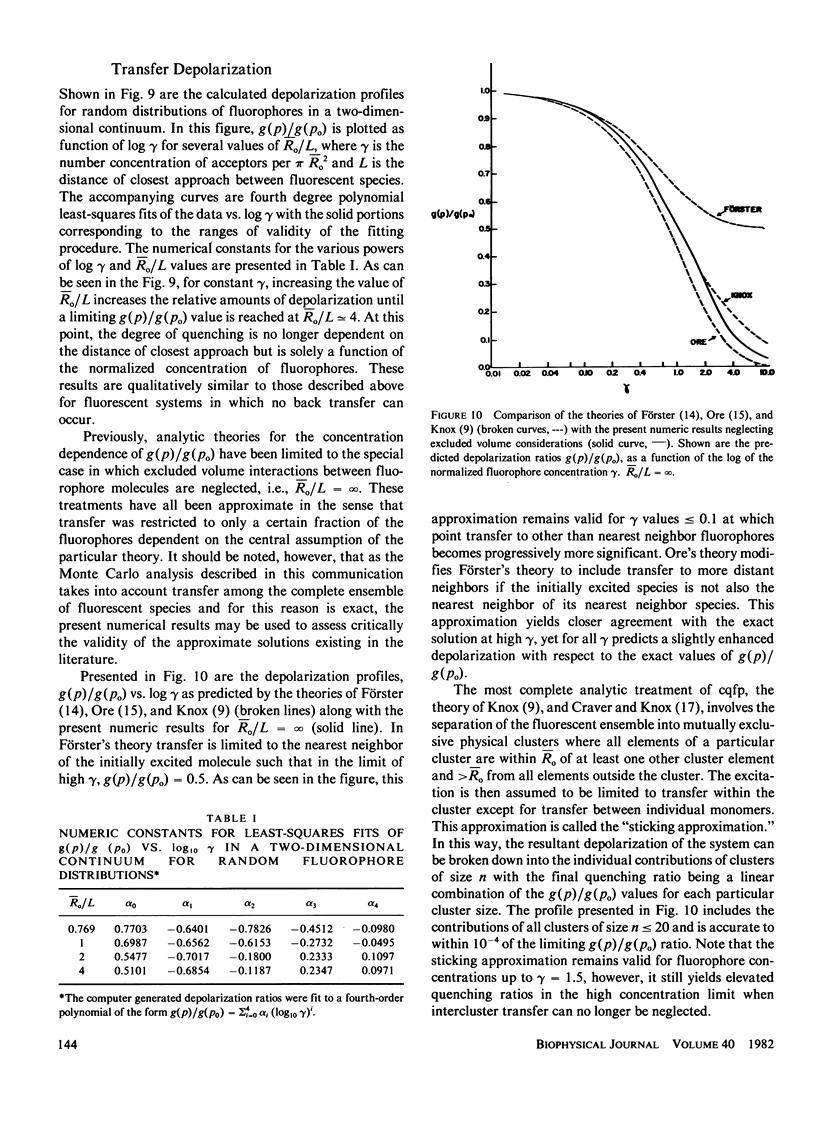
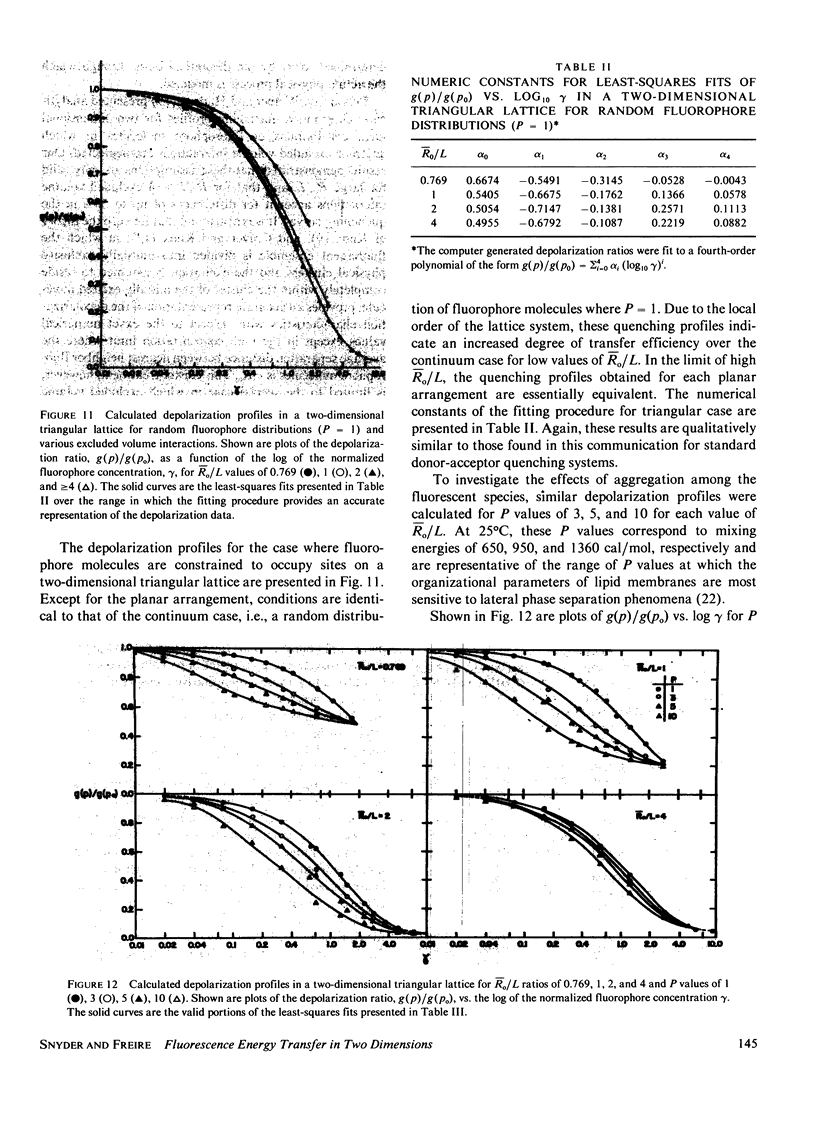
Abstract
A method of Monte Carlo calculations has been applied to the problem of fluorescence energy transfer in two dimensions in order to provide a quantitative measure of the effects of nonideal mixing of lipid and protein molecules on the quenching profiles of membrane systems. These numerical techniques permit the formulation of a detailed set of equations that describes in a precise manner the quenching and depolarization properties of planar donor-acceptor distributions as a function of specific spectroscopic and organizational parameters. Because of the exact nature of the present numeric method, these results are used to evaluate critically the validity of previous approximate treatments existing in the literature. This method is also used to examine the effects of excluded volume interactions and distinct lattice structures on the expected transfer efficiencies. As a specific application, representative quenching profiles for protein-lipid mixtures, in which donor groups are covalently linked to the protein molecules and acceptor species are randomly distributed within lipid domains, have been obtained. It is found that the existence of phase-separated protein domains gives rise to a shielding effect that significantly decreases the transfer efficiencies with respect to those expected for an ideal distribution of protein molecules. The results from the present numerical study indicate that the experimental application of fluorescence energy transfer measurements in multicomponent membrane systems can be used to obtain organizational parameters that accurately reflect the lateral distribution of protein and lipid molecules within the bilayer membrane.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey T. G., Hammes G. G. Calculation on fluorescence resonance energy transfer on surfaces. Biophys J. 1980 Dec;32(3):1023–1035. doi: 10.1016/S0006-3495(80)85033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissing S., Jesaitis A. J., Fortes P. A. Fluorescence labeling of the human erythrocyte anion transport system. Biochim Biophys Acta. 1979 May 3;553(1):66–83. doi: 10.1016/0005-2736(79)90031-2. [DOI] [PubMed] [Google Scholar]
- Freire E., Snyder B. Estimation of the lateral distribution of molecules in two-component lipid bilayers. Biochemistry. 1980 Jan 8;19(1):88–94. doi: 10.1021/bi00542a014. [DOI] [PubMed] [Google Scholar]
- Freire E., Snyder B. Monte Carlo studies of the lateral organization of molecules in two-component lipid bilayers. Biochim Biophys Acta. 1980 Aug 14;600(3):643–654. doi: 10.1016/0005-2736(80)90468-x. [DOI] [PubMed] [Google Scholar]
- Freire E., Snyder B. Quantitative characterization of the lateral distribution of membrane proteins within the lipid bilayer. Biophys J. 1982 Mar;37(3):617–624. [PMC free article] [PubMed] [Google Scholar]
- Fung B. K., Stryer L. Surface density determination in membranes by fluorescence energy transfer. Biochemistry. 1978 Nov 28;17(24):5241–5248. doi: 10.1021/bi00617a025. [DOI] [PubMed] [Google Scholar]
- Hahn L. H., Hammes G. G. Structural mapping of aspartate transcarbamoylase by fluorescence energy-transfer measurements: determination of the distance between catalytic sites of different subunits. Biochemistry. 1978 Jun 13;17(12):2423–2429. doi: 10.1021/bi00605a027. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Tecoma E. S., Wolber P. K., Hudson B. S., Wickner W. T., Simoni R. D. Protein-lipid interactions. Studies of the M13 coat protein in dimyristoylphosphatidylcholine vesicles using parinaric acid. Biochemistry. 1979 Dec 25;18(26):5874–5880. doi: 10.1021/bi00593a018. [DOI] [PubMed] [Google Scholar]
- Knox R. S. On the theory of trapping of excitation in the photosynthetic unit. J Theor Biol. 1968 Nov;21(2):244–259. doi: 10.1016/0022-5193(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Fleming P. J., Strittmatter P. Intramembrane positions of membrane-bound chromophores determined by excitation energy transfer. Biochemistry. 1979 Nov 27;18(24):5450–5457. doi: 10.1021/bi00591a030. [DOI] [PubMed] [Google Scholar]
- Lee A. G. Segregation of chlorophyll a incorporated into lipid bilayers. Biochemistry. 1975 Oct 7;14(20):4397–4402. doi: 10.1021/bi00691a009. [DOI] [PubMed] [Google Scholar]
- Rogers J., Lee A. G., Wilton D. C. The organisation of cholesterol and ergosterol in lipid bilayers based on studies using non-perturbing fluorescent sterol probes. Biochim Biophys Acta. 1979 Mar 23;552(1):23–37. doi: 10.1016/0005-2736(79)90243-8. [DOI] [PubMed] [Google Scholar]
- Snyder B., Freire E. Compositional domain structure in phosphatidylcholine--cholesterol and sphingomyelin--cholesterol bilayers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4055–4059. doi: 10.1073/pnas.77.7.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Veatch W., Stryer L. The dimeric nature of the gramicidin A transmembrane channel: conductance and fluorescence energy transfer studies of hybrid channels. J Mol Biol. 1977 Jun 15;113(1):89–102. doi: 10.1016/0022-2836(77)90042-0. [DOI] [PubMed] [Google Scholar]
- WEBER G. Fluorescence-polarization spectrum and electronic-energy transfer in tyrosine, tryptophan and related compounds. Biochem J. 1960 May;75:335–345. doi: 10.1042/bj0750335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Daniel E. Cooperative effects in binding by bovine serum albumin. II. The binding of 1-anilino-8-naphthalenesulfonate. Polarization of the ligand fluorescence and quenching of the protein fluorescence. Biochemistry. 1966 Jun;5(6):1900–1907. doi: 10.1021/bi00870a017. [DOI] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys J. 1979 Nov;28(2):197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


