Abstract
Discrete sarcomere lengths have been determined from dynamically contracting isolated cardiac cells with a high-speed, high-resolution direct optical imaging system. Calcium-tolerant cardiac cells from the rat are isolated by perfusion with collagenase and hyaluronidase. Individual sarcomere lengths can be determined by directly imaging the cell's striation pattern onto a solid-state charge-coupled device (CCD) detector interfaced with a digital computer. The precision of detection in a real light microscopic optical system is discussed in relation to the type of image detector, optical contract enhancement techniques, and digital image processing. The optical performance of the direct striation pattern image apparatus has been determined empirically with test grids under standard bright-field and Nomarski-differential interference contrast (DIC) conditions for application to real muscle imaging. Discrete striation positions of isolated cells have been detected and followed with high precision during phasic contraction-relaxation cycles down to average sarcomere lengths as short as 1.43 +/- 0.053 microns. The maximum rates of contraction and relaxation are rapid and synchronous in time course along the length of the cell. These results indicate that direct optical imaging can provide an accurate means to monitor discrete striations and sarcomere lengths along the length of Ca2+-tolerant heart cells.
Full text
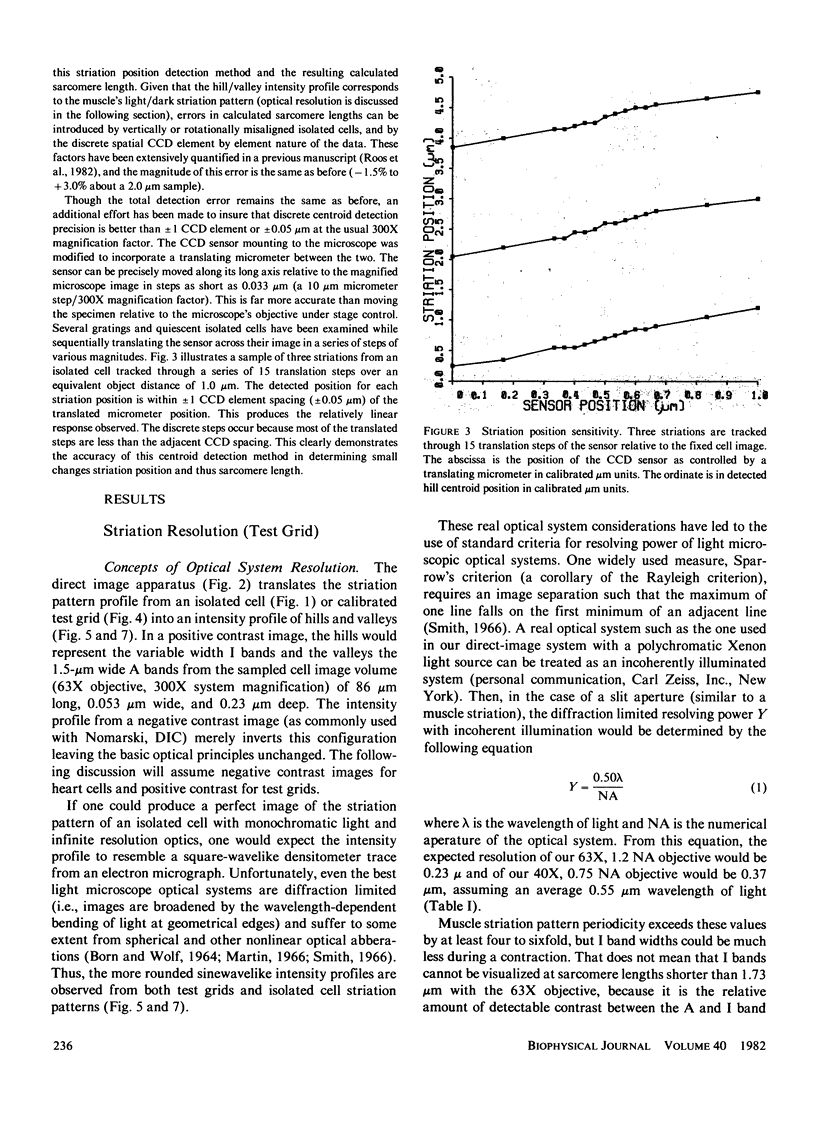
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Allen N. S., Travis J. L. Video-enhanced contrast, differential interference contrast (AVEC-DIC) microscopy: a new method capable of analyzing microtubule-related motility in the reticulopodial network of Allogromia laticollaris. Cell Motil. 1981;1(3):291–302. doi: 10.1002/cm.970010303. [DOI] [PubMed] [Google Scholar]
- Allen R. D., David G. B., Nomarski G. The zeiss-Nomarski differential interference equipment for transmitted-light microscopy. Z Wiss Mikrosk. 1969 Nov;69(4):193–221. [PubMed] [Google Scholar]
- Allen R. D., Travis J. L., Allen N. S., Yilmaz H. Video-enhanced contrast polarization (AVEC-POL) microscopy: a new method applied to the detection of birefringence in the motile reticulopodial network of Allogromia laticollaris. Cell Motil. 1981;1(3):275–289. doi: 10.1002/cm.970010302. [DOI] [PubMed] [Google Scholar]
- Brady A. J., Tan S. T., Ricchiuti N. V. Contractile force measured in unskinned isolated adult rat heart fibres. Nature. 1979 Dec 13;282(5740):728–729. doi: 10.1038/282728a0. [DOI] [PubMed] [Google Scholar]
- Cleworth D. R., Edman K. A. Changes in sarcomere length during isometric tension development in frog skeletal muscle. J Physiol. 1972 Dec;227(1):1–17. doi: 10.1113/jphysiol.1972.sp010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clerck N. M., Claes V. A., Van Ocken E. R., Brutsaert D. L. Sarcomere distribution patterns in single cardiac cells. Biophys J. 1981 Jul;35(1):237–242. doi: 10.1016/S0006-3495(81)84784-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Techniques of skinned cardiac cells and of isolated cardiac fibers with disrupted sarcolemmas with reference to the effects of catecholamines and of caffeine. Recent Adv Stud Cardiac Struct Metab. 1976;9:1–94. [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J Physiol. 1966 May;184(1):170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Measurement of the striations of isolated muscle fibres with the interference microscope. J Physiol. 1958 Dec 30;144(3):403–425. doi: 10.1113/jphysiol.1958.sp006110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Forletti D., Wittenberg B. A. Uniform sarcomere shortening behavior in isolated cardiac muscle cells. J Gen Physiol. 1980 Nov;76(5):587–607. doi: 10.1085/jgp.76.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. W., Pollack G. H. Myocardial sarcomere dynamics during isometric contraction. J Physiol. 1975 Oct;251(3):627–643. doi: 10.1113/jphysiol.1975.sp011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolini P. J., Roos K. P., Baskin R. J. Light diffraction studies of sarcomere dynamics in single skeletal muscle fibers. Biophys J. 1977 Nov;20(2):221–232. doi: 10.1016/S0006-3495(77)85545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack G. H., Iwazumi T., ter Keurs H. E., Shibata E. F. Sarcomere shortening in striated muscle occurs in stepwise fashion. Nature. 1977 Aug 25;268(5622):757–759. doi: 10.1038/268757a0. [DOI] [PubMed] [Google Scholar]
- Powell T., Terrar D. A., Twist V. W. Electrical properties of individual cells isolated from adult rat ventricular myocardium. J Physiol. 1980 May;302:131–153. doi: 10.1113/jphysiol.1980.sp013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieser G., Sabbadini R., Paolini P., Fry M., Inesi G. Sarcomere motion in isolated cardiac cells. Am J Physiol. 1979 Jan;236(1):C70–C77. doi: 10.1152/ajpcell.1979.236.1.C70. [DOI] [PubMed] [Google Scholar]
- Roos K. P., Baskin R. J., Lieber R. L., Cline J. W., Paolini P. J. Digital data acquisition and analysis of striated muscle diffraction patterns with a direct memory access microprocessor system. Rev Sci Instrum. 1980 Jun;51(6):762–767. doi: 10.1063/1.1136308. [DOI] [PubMed] [Google Scholar]
- Roos K. P., Brady A. J., Tan S. T. Direct measurement of sarcomere length from isolated cardiac cells. Am J Physiol. 1982 Jan;242(1):H68–H78. doi: 10.1152/ajpheart.1982.242.1.H68. [DOI] [PubMed] [Google Scholar]
- Rüdel R., Zite-Ferenczy F. Interpretation of light diffraction by cross-striated muscle as Bragg reflexion of light by the lattice of contractile proteins. J Physiol. 1979 May;290(2):317–330. doi: 10.1113/jphysiol.1979.sp012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter R. J., Berns M. W. Computer-enhanced video microscopy: digitally processed microscope images can be produced in real time. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6927–6931. doi: 10.1073/pnas.78.11.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg B. A., Robinson T. F. Oxygen requirements, morphology, cell coat and membrane permeability of calcium-tolerant myocytes from hearts of adult rats. Cell Tissue Res. 1981;216(2):231–251. doi: 10.1007/BF00233618. [DOI] [PubMed] [Google Scholar]




