Abstract
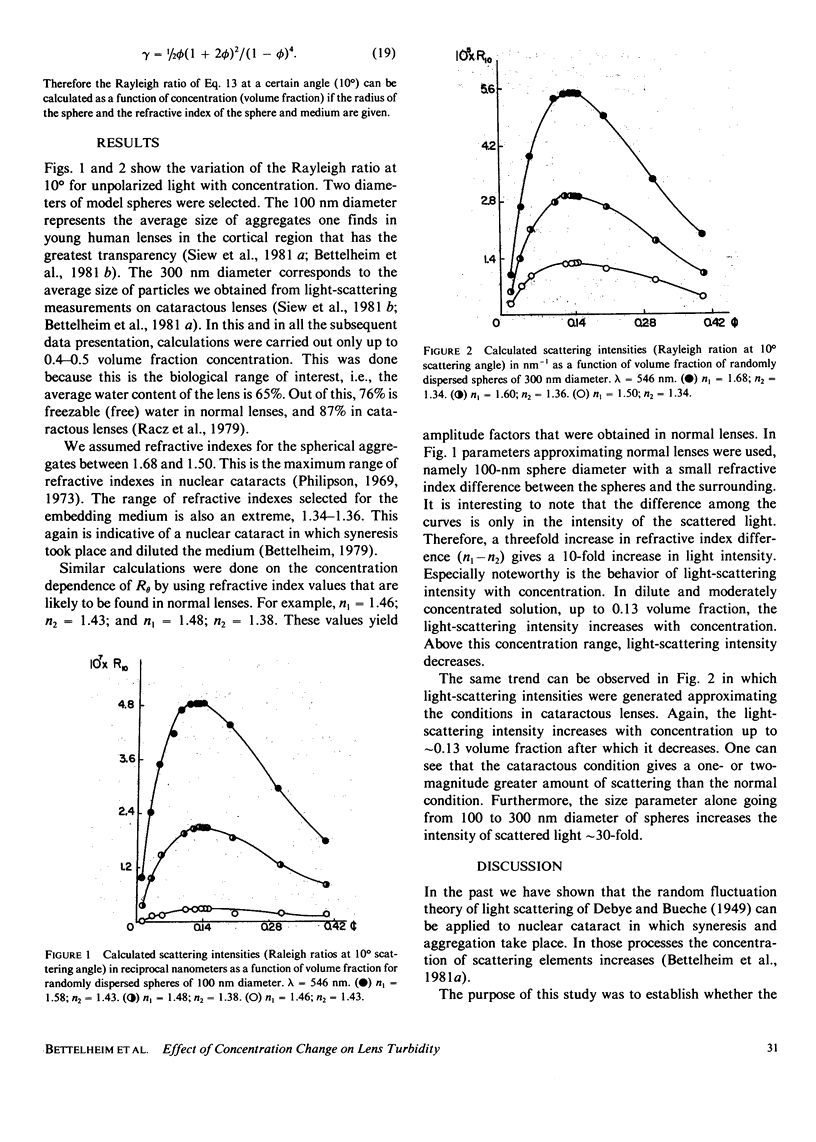
Theoretical calculations were performed to predict how the light scattering intensity would change with changes in concentration in the gel state. The theory of light scattering was applied to a random distribution of hard spheres. The spherical particles with constant diameter were embedded in a medium having a different refractive index. The light-scattering intensities obtained as a function of concentration showed that in dilute solutions the scattered light intensity increases with concentration. However, in concentrated solution greater than 0.1 or 0.2 volume fraction, the light-scattering intensity decreases with increase in concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettelheim F. A., Bettelheim A. A. Small-angle light scattering studies on xylose cataract formation in bovine lenses. Invest Ophthalmol Vis Sci. 1978 Sep;17(9):896–902. [PubMed] [Google Scholar]
- Bettelheim F. A. On the optical anisotropy of lens fiber cells. Exp Eye Res. 1975 Sep;21(3):231–234. doi: 10.1016/0014-4835(75)90093-7. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A., Paunovic M. Light scattering of normal human lens I. Application of random density and orientation fluctuation theory. Biophys J. 1979 Apr;26(1):85–99. doi: 10.1016/S0006-3495(79)85237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim F. A., Siew E. L., Chylack L. T., Jr Studies on human cataracts. III. Structural elements in nuclear cataracts and their contribution to the turbidity. Invest Ophthalmol Vis Sci. 1981 Mar;20(3):348–354. [PubMed] [Google Scholar]
- Bettelheim F. A., Siew E. L., Shyne S., Farnsworth P., Burke P. A comparative study of human lens by light scattering and scanning electron microscopy. Exp Eye Res. 1981 Jan;32(1):125–129. doi: 10.1016/s0014-4835(81)80045-0. [DOI] [PubMed] [Google Scholar]
- Bettelheim F. A. Syneresis and its possible role in cataractogenesis. Exp Eye Res. 1979 Feb;28(2):189–197. doi: 10.1016/0014-4835(79)90130-1. [DOI] [PubMed] [Google Scholar]
- Chylack L. T., Jr, Bettelheim F. A., Tung W. H. Studies on human cataracts. I. Evaluation of techniques of human cataract preservation after extraction. Invest Ophthalmol Vis Sci. 1981 Mar;20(3):327–333. [PubMed] [Google Scholar]
- Hoenders H. J., van Kamp G. J. Eye lens development and aging processes of crystallins. Acta Morphol Neerl Scand. 1972 Nov;10(3):215–221. [PubMed] [Google Scholar]
- Ishimoto C., Goalwin P. W., Sun S. T., Nishio I., Tanaka T. Cytoplasmic phase separation in formation of galactosemic cataract in lenses of young rats. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4414–4416. doi: 10.1073/pnas.76.9.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata S., Kinoshita J. H. Mechanism of development of hereditary cataract in mice. Invest Ophthalmol. 1971 Jul;10(7):504–512. [PubMed] [Google Scholar]
- Jedziniak J. A., Kinoshita J. H., Yates E. M., Benedek G. B. The concentration and localization of heavy molecular weight aggregates in aging normal and cataractous human lenses. Exp Eye Res. 1975 Apr;20(4):367–369. doi: 10.1016/0014-4835(75)90118-9. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Nicoli D. F., Baram H., Benedek G. B. Quantitative verification of the existence of high molecular weight protein aggregates in the intact normal human lens by light-scattering spectroscopy. Invest Ophthalmol Vis Sci. 1978 Jan;17(1):51–57. [PubMed] [Google Scholar]
- Kinoshita J. H. Cataracts in galactosemia. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1965 Oct;4(5):786–799. [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Philipson B. Changes in the lens related to the reduction of transparency. Exp Eye Res. 1973 Jun;16(1):29–39. doi: 10.1016/0014-4835(73)90234-0. [DOI] [PubMed] [Google Scholar]
- Rácz P., Tompa K., Pócsik I. The state of water in normal and senile cataractous lenses studied by nuclear magnetic resonance. Exp Eye Res. 1979 Feb;28(2):129–135. doi: 10.1016/0014-4835(79)90125-8. [DOI] [PubMed] [Google Scholar]
- Siew E. L., Bettelheim F. A., Chylack L. T., Jr, Tung W. H. Studies on human cataracts. II. Correlation between the clinical description and the light-scattering parameters of human cataracts. Invest Ophthalmol Vis Sci. 1981 Mar;20(3):334–347. [PubMed] [Google Scholar]
- Siew E. L., Opalecky D., Bettelheim F. A. Light scattering of normal human lens. II. Age dependence of the light scattering parameters. Exp Eye Res. 1981 Dec;33(6):603–614. doi: 10.1016/s0014-4835(81)80100-5. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., Berger H. The quaternary structure of bovine alpha-crystallin. Size and shape studies by sedimentation, small-angle X-ray scattering and quasi-elastic light scattering. Eur J Biochem. 1978 Nov 15;91(2):397–405. doi: 10.1111/j.1432-1033.1978.tb12692.x. [DOI] [PubMed] [Google Scholar]
- Spector A. Aggregation of a-crystallin and its possible relationship to cataract formation. Isr J Med Sci. 1972 Aug-Sep;8(8):1577–1582. [PubMed] [Google Scholar]
- TROKEL S. The physical basis for transparency of the crystalline lens. Invest Ophthalmol. 1962 Aug;1:493–501. [PubMed] [Google Scholar]


