Abstract
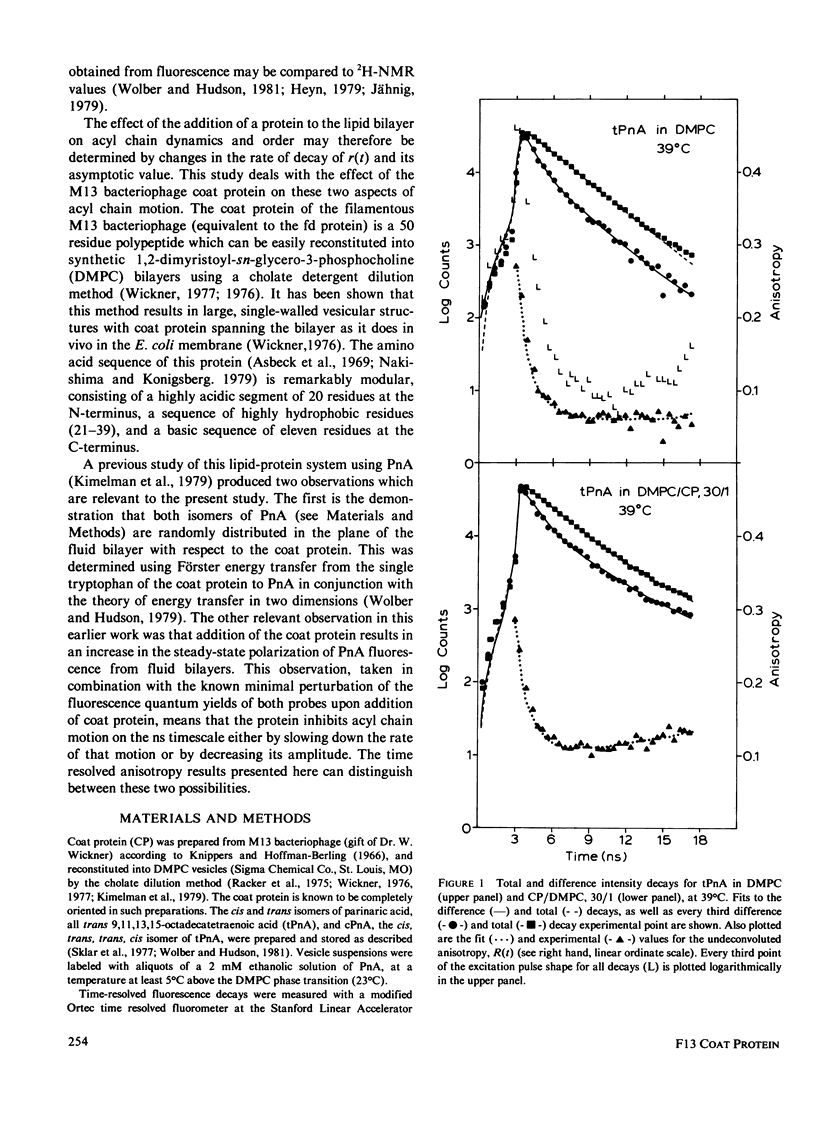
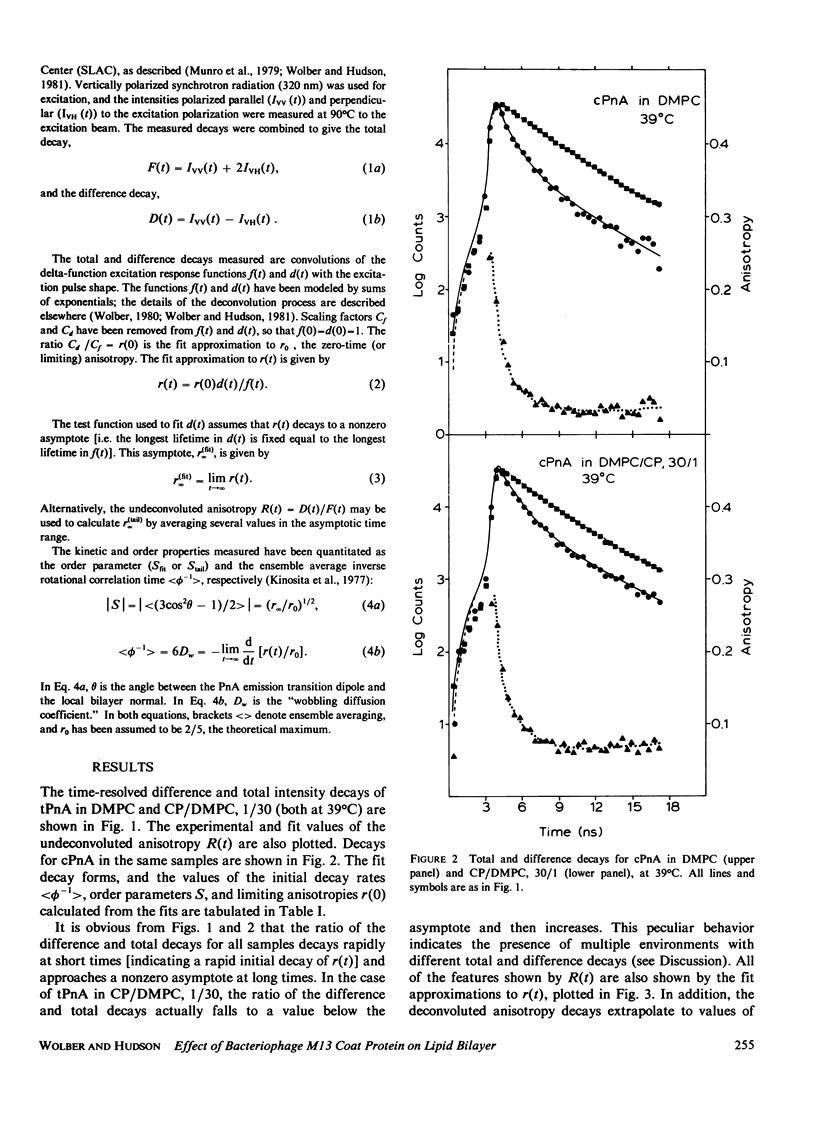
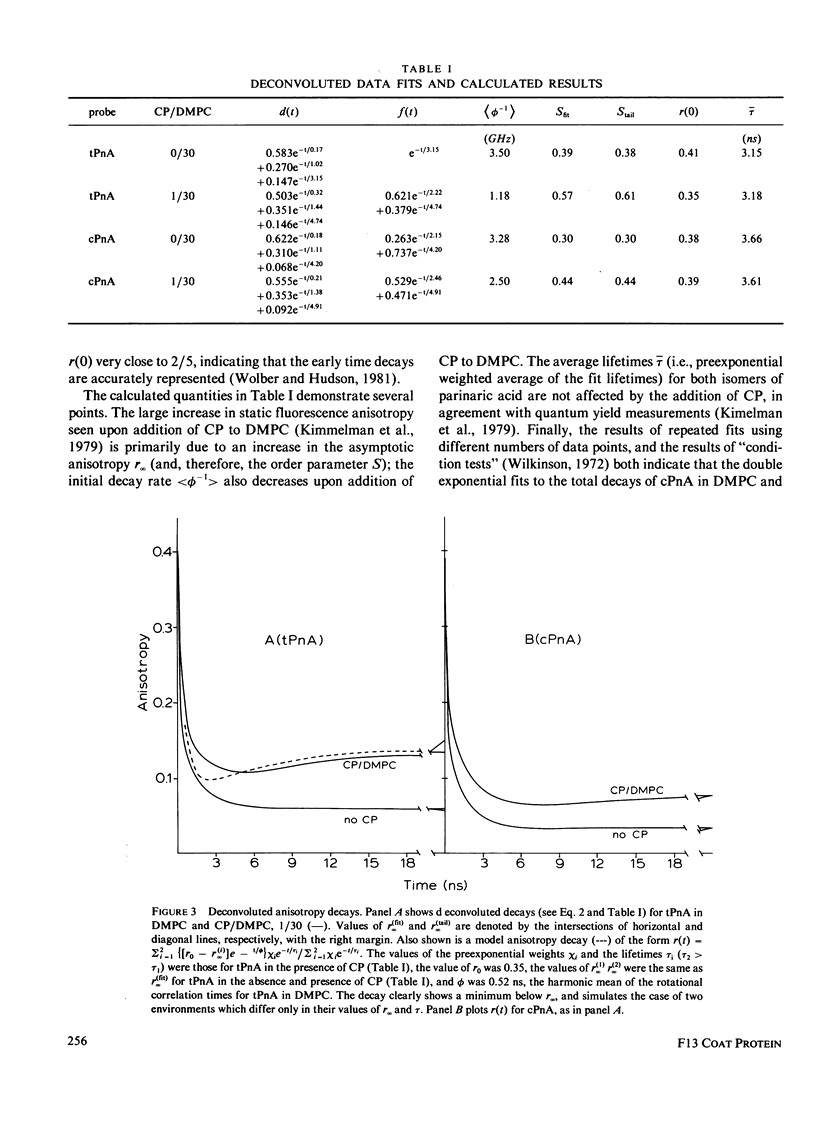
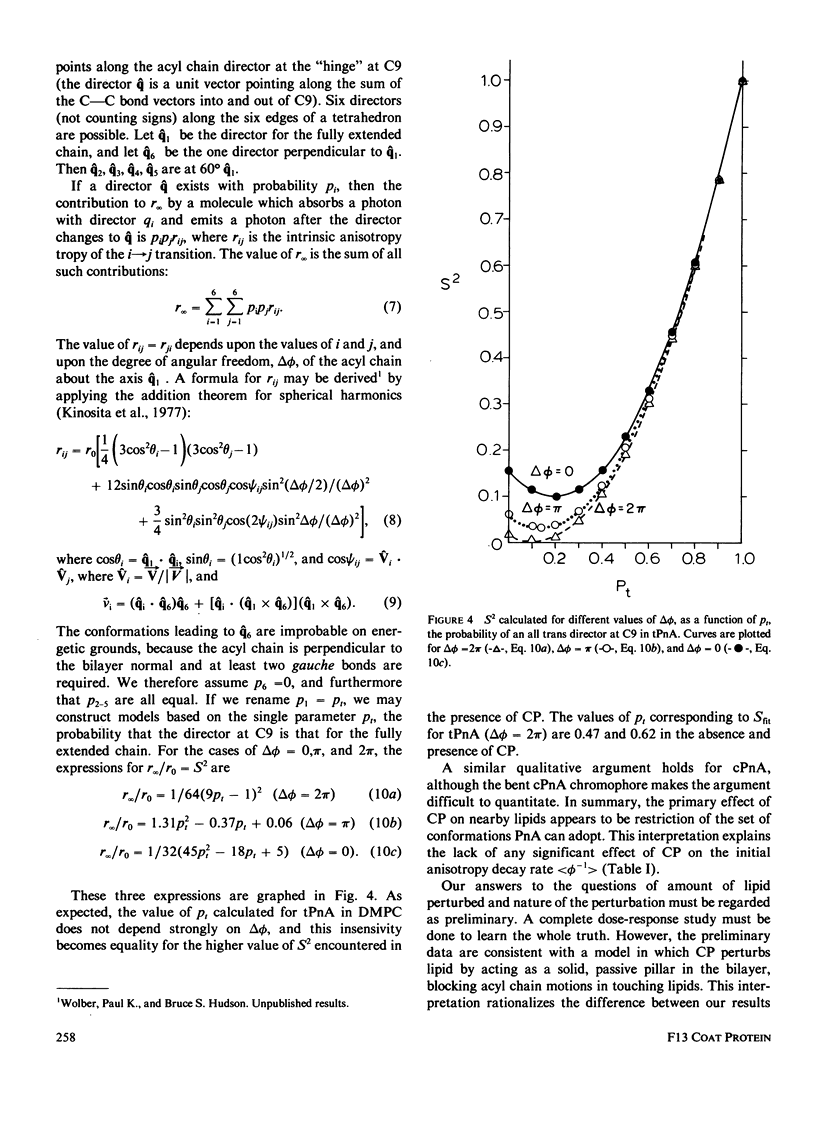
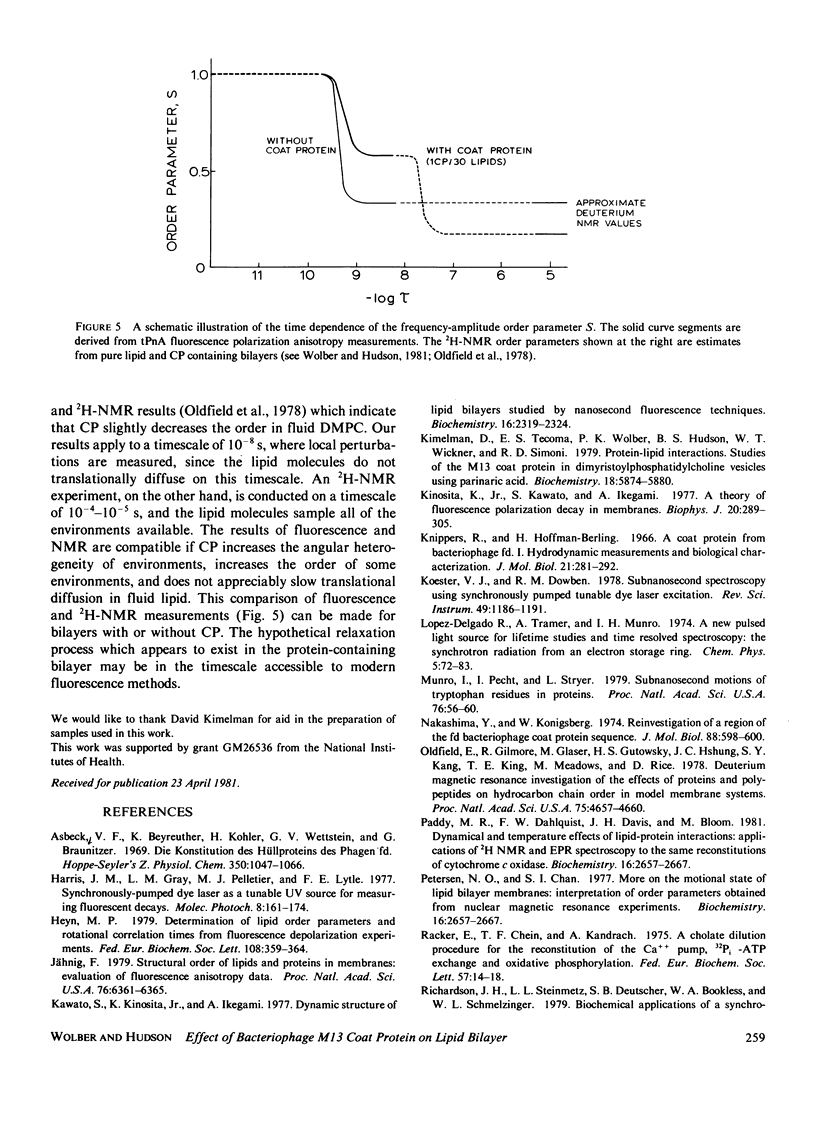
Nanosecond fluorescence polarization anisotropy decay is used to determine the effect of the bacteriophage M13 coat protein on lipid bilayer acyl chain dynamics and order. The fluorescent acyl chain analogues cis- and trans-parinaric acid were used to determine the rate and extent of the angular motion of acyl chains in liquid crystalline (39 degrees C) dimyristoylphosphatidylcholine bilayers free of coat protein or containing the coat protein at a protein:lipid ratio of 1:30. Subnanosecond time resolution was obtained by using synchrotron radiation as the excitation source for single photon counting detection. Previous measurements of Förster energy transfer from coat protein tryptophan to cis- or trans-parinaric acid have shown that these probes are randomly distributed in the bilayer with respect to the protein. The anisotropy decay observed for pure bilayers has the form of a rapid drop, followed by a nonzero constant region extending from roughly 3 ns to at least 12 ns. The magnitude of the anisotropy in the plateau region is simply related to the acyl chain order parameter. The effect of the M13 coat protein is to increase the acyl chain order parameter significantly while having only a small effect on the rate of angular relaxation. This behavior is rationalized in terms of a simple microscopic model. The order parameters for pure lipid and coat protein containing bilayers are compared to 2H-NMR values.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbeck F., Beyreuther K., Köhler H., von Wettstein G., Braunitzer G. Virusproteine, IV. Die Konstitution des Hüllproteins des Phagen fd. Hoppe Seylers Z Physiol Chem. 1969 Sep;350(9):1047–1066. [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato S., Kinosita K., Jr, Ikegami A. Dynamic structure of lipid bilayers studied by nanosecond fluorescence techniques. Biochemistry. 1977 May 31;16(11):2319–2324. doi: 10.1021/bi00630a002. [DOI] [PubMed] [Google Scholar]
- Kimelman D., Tecoma E. S., Wolber P. K., Hudson B. S., Wickner W. T., Simoni R. D. Protein-lipid interactions. Studies of the M13 coat protein in dimyristoylphosphatidylcholine vesicles using parinaric acid. Biochemistry. 1979 Dec 25;18(26):5874–5880. doi: 10.1021/bi00593a018. [DOI] [PubMed] [Google Scholar]
- Kinosita K., Jr, Kawato S., Ikegami A. A theory of fluorescence polarization decay in membranes. Biophys J. 1977 Dec;20(3):289–305. doi: 10.1016/S0006-3495(77)85550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Hoffmann-Berling H. A coat protein from bacteriophage fd. I. Hydrodynamic measurements and biological characterization. J Mol Biol. 1966 Nov 14;21(2):281–292. doi: 10.1016/0022-2836(66)90099-4. [DOI] [PubMed] [Google Scholar]
- Munro I., Pecht I., Stryer L. Subnanosecond motions of tryptophan residues in proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):56–60. doi: 10.1073/pnas.76.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Konigsberg W. Reinvestigation of a region of the fd bacteriophage coat protein sequence. J Mol Biol. 1974 Sep 25;88(3):598–600. doi: 10.1016/0022-2836(74)90410-0. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Gilmore R., Glaser M., Gutowsky H. S., Hshung J. C., Kang S. Y., King T. E., Meadows M., Rice D. Deuterium nuclear magnetic resonance investigation of the effects of proteins and polypeptides on hydrocarbon chain order in model membrane systems. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4657–4660. doi: 10.1073/pnas.75.10.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N. O., Chan S. I. More on the motional state of lipid bilayer membranes: interpretation of order parameters obtained from nuclear magnetic resonance experiments. Biochemistry. 1977 Jun 14;16(12):2657–2667. doi: 10.1021/bi00631a012. [DOI] [PubMed] [Google Scholar]
- Racker E., Chien T. F., Kandrach A. A cholate-dilution procedure for the reconstitution of the Ca++ pump, 32Pi--ATP exchange, and oxidative phosphorylation. FEBS Lett. 1975 Sep 1;57(1):14–18. doi: 10.1016/0014-5793(75)80141-4. [DOI] [PubMed] [Google Scholar]
- Richardson J. H., Steinmetz L. L., Deutscher S. B., Bookless W. A., Schmelzinger W. L. Biochemical applications of a synchronously pumped krypton ion dye laser fluorescence system. Anal Biochem. 1979 Aug;97(1):17–23. doi: 10.1016/0003-2697(79)90321-x. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as fluorescent probes: synthetic phospholipid membrane studies. Biochemistry. 1977 Mar 8;16(5):819–828. doi: 10.1021/bi00624a002. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Hudson B. S., Simoni R. D. Conjugated polyene fatty acids as membrane probes: preliminary characterization. Proc Natl Acad Sci U S A. 1975 May;72(5):1649–1653. doi: 10.1073/pnas.72.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W. T. Role of hydrophobic forces in membrane protein asymmetry. Biochemistry. 1977 Jan 25;16(2):254–258. doi: 10.1021/bi00621a015. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. An analytic solution to the Förster energy transfer problem in two dimensions. Biophys J. 1979 Nov;28(2):197–210. doi: 10.1016/S0006-3495(79)85171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolber P. K., Hudson B. S. Fluorescence lifetime and time-resolved polarization anisotropy studies of acyl chain order and dynamics in lipid bilayers. Biochemistry. 1981 May 12;20(10):2800–2810. doi: 10.1021/bi00513a015. [DOI] [PubMed] [Google Scholar]


