Abstract
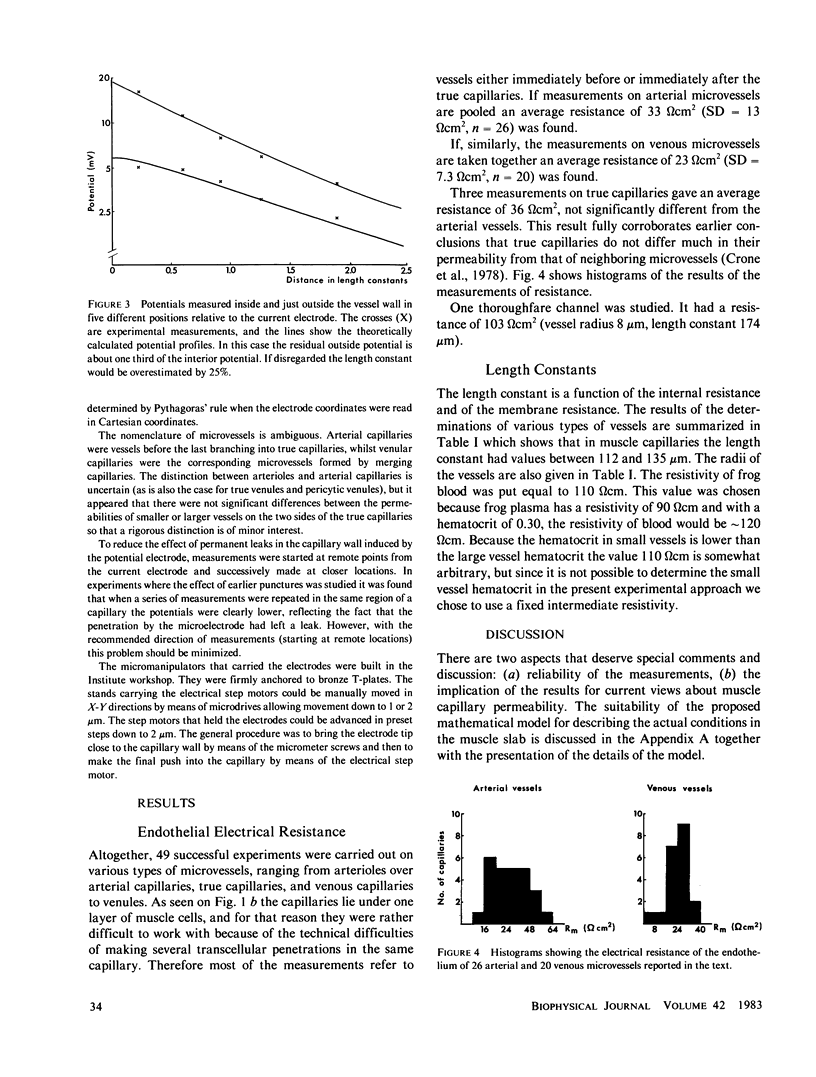
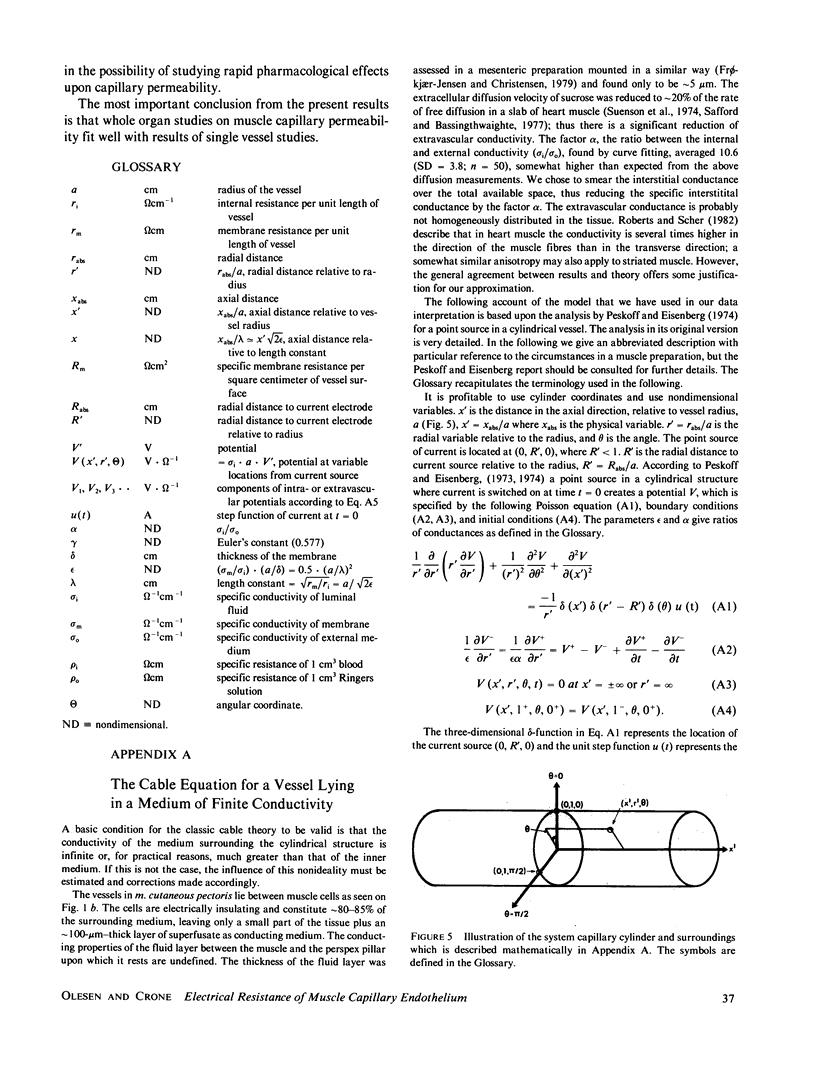
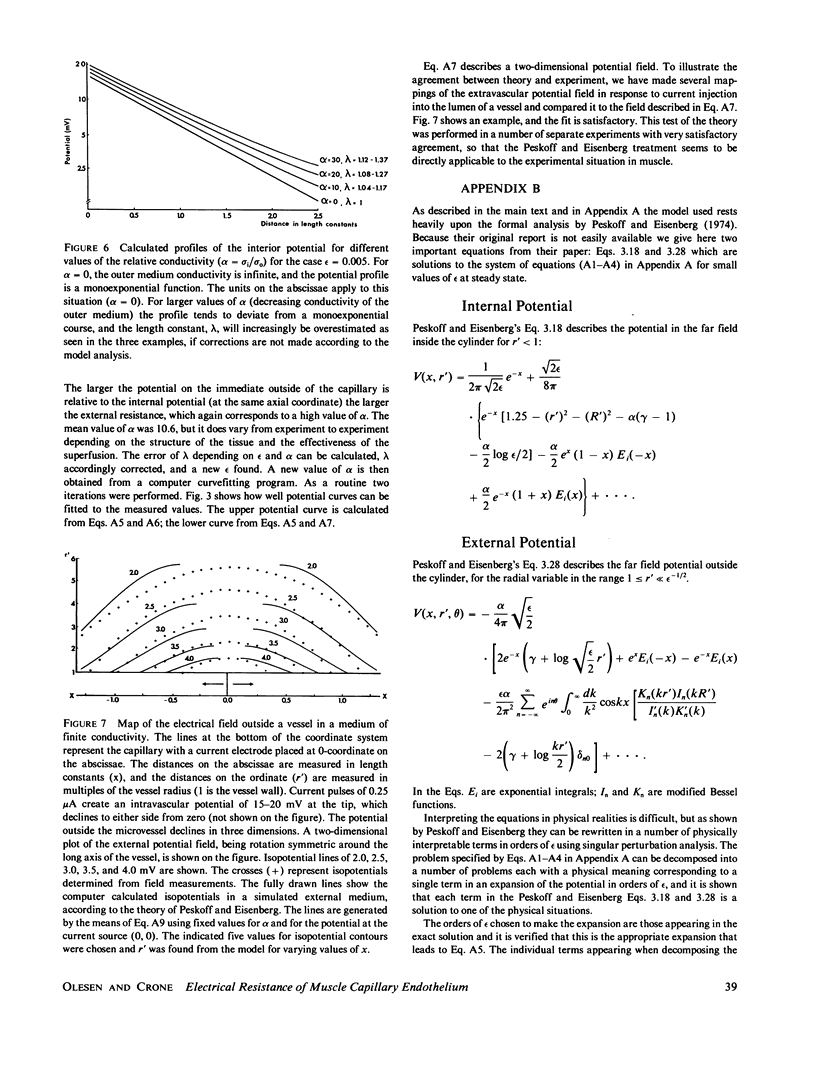
A recently developed technique for in vivo determination of the electrical resistance of vascular endothelium in microvessels was applied to the vessels in a thin frog muscle, m. cutaneus pectoris. The technique consists of injection of current via a glass micropipette into a capillary and measurement of the resulting intra- and extravascular potential profiles with another micropipette placed at various distances from the current source. The theory of Peskoff and Eisenberg (1974) was used to handle the problems arising from distributed extravascular resistances and was experimentally shown to describe the external field satisfactorily. With this extension of one-dimensional cable theory the specific electrical resistance of arterial microvessels was 33 omega cm2 and of venous capillaries 23 omega cm2. The "length constants" were 135 and 112 micrometers, respectively. If results from arterial and venous vessels are taken together, the ionic permeabilities at 20 degrees C were PNa = 3.9 X 10(-5) cm X s-1, PK = 5.7 X 10(-5) cm X s-1, PCl = 5.9 X 10(-5) cm X s-1 and PHCO3 = 3.4 X 10(-5) cm X s-1. These figures agree with figures for capillary permeability obtained in tracer experiments on whole muscle. The study bridges a gap between single capillary and whole organ techniques with the conclusion that the two different approaches lead to similar results in muscle capillaries.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a Single End-Organ. J Physiol. 1926 Apr 23;61(2):151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulpaep E. L. Permeability changes of the proximal tubule of Necturus during saline loading. Am J Physiol. 1972 Mar;222(3):517–531. doi: 10.1152/ajplegacy.1972.222.3.517. [DOI] [PubMed] [Google Scholar]
- Bundgaard M., Frøkjaer-Jensen J. Functional aspects of the ultrastructure of terminal blood vessels: a qualitative study on consecutive segments of the frog mesenteric microvasculature. Microvasc Res. 1982 Jan;23(1):1–30. doi: 10.1016/0026-2862(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Casley-Smith J. R., Green H. S., Harris J. L., Wadey P. J. The quantitative morphology of skeletal muscle capillaries in relation to permeability. Microvasc Res. 1975 Jul;10(1):43–64. doi: 10.1016/0026-2862(75)90019-9. [DOI] [PubMed] [Google Scholar]
- Crone C., Christensen O. Electrical resistance of a capillary endothelium. J Gen Physiol. 1981 Apr;77(4):349–371. doi: 10.1085/jgp.77.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Frøkjaer-Jensen J., Friedman J. J., Christensen O. The permeability of single capillaries to potassium ions. J Gen Physiol. 1978 Feb;71(2):195–220. doi: 10.1085/jgp.71.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C., Olesen S. P. Electrical resistance of brain microvascular endothelium. Brain Res. 1982 Jun 3;241(1):49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C., Mason J. C. Osmotic reflextion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of the frog mesentery. J Physiol. 1976 Oct;261(2):319–336. doi: 10.1113/jphysiol.1976.sp011561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E. Permeability coefficients of the capillary wall to low molecular weight hydrophilic solutes measured in single perfused capillaries of frog mesentery. Microvasc Res. 1979 May;17(3 Pt 1):290–308. doi: 10.1016/s0026-2862(79)80005-9. [DOI] [PubMed] [Google Scholar]
- Durán W. N. Effects of muscle contraction and of adenosine on capillary transport and microvascular flow in dog skeletal muscle. Circ Res. 1977 Nov;41(5):642–647. doi: 10.1161/01.res.41.5.642. [DOI] [PubMed] [Google Scholar]
- Frøkjaer-Jensen J., Christensen O. Potassium permeability of the mesothelium of the frog mesentery. Acta Physiol Scand. 1979 Feb;105(2):228–238. doi: 10.1111/j.1748-1716.1979.tb06335.x. [DOI] [PubMed] [Google Scholar]
- Frøkjaer-Jensen J. Permeability of single muscle capillaries to potassium ions. Microvasc Res. 1982 Sep;24(2):168–183. doi: 10.1016/0026-2862(82)90054-1. [DOI] [PubMed] [Google Scholar]
- Gore R. W. Fluid exchange across single capillaries in rat intestinal muscle. Am J Physiol. 1982 Feb;242(2):H268–H287. doi: 10.1152/ajpheart.1982.242.2.H268. [DOI] [PubMed] [Google Scholar]
- Guller B., Yipintsoi T., Orvis A. L., Bassingthwaighte J. B. Myocardial sodium extraction at varied coronary flows in the dog. Estimation of capillary permeability of residue and outflow detection. Circ Res. 1975 Sep;37(3):359–378. doi: 10.1161/01.res.37.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudlická O., Zweifach B. W., Tyler K. R. Capillary recruitment and flow velocity in skeletal muscle after contractions. Microvasc Res. 1982 Mar;23(2):201–213. doi: 10.1016/0026-2862(82)90065-6. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919 May 20;52(6):409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R. Passage of molecules through capillary wals. Physiol Rev. 1953 Jul;33(3):387–423. doi: 10.1152/physrev.1953.33.3.387. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., RENKIN E. M., BORRERO L. M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951 Oct;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Paaske W. P. Capillary permeability in skeletal muscle. Acta Physiol Scand. 1977 Sep;101(1):1–14. doi: 10.1111/j.1748-1716.1977.tb05977.x. [DOI] [PubMed] [Google Scholar]
- Perry M. A. Capillary filtration and permeability coefficients calculated from measurements of interendothelial cell junctions in rabbit lung and skeletal muscle. Microvasc Res. 1980 Mar;19(2):142–157. doi: 10.1016/0026-2862(80)90036-9. [DOI] [PubMed] [Google Scholar]
- Peskoff A., Eisenberg R. S. Interpretation of some microelectrode measurements of electrical properties of cells. Annu Rev Biophys Bioeng. 1973;2:65–79. doi: 10.1146/annurev.bb.02.060173.000433. [DOI] [PubMed] [Google Scholar]
- Renkin E. M., Hudlická O., Sheehan R. M. Influence of metabolic vasodilatation on blood-tissue diffusion in skeletal muscle. Am J Physiol. 1966 Jul;211(1):87–98. doi: 10.1152/ajplegacy.1966.211.1.87. [DOI] [PubMed] [Google Scholar]
- Roberts D. E., Scher A. M. Effect of tissue anisotropy on extracellular potential fields in canine myocardium in situ. Circ Res. 1982 Mar;50(3):342–351. doi: 10.1161/01.res.50.3.342. [DOI] [PubMed] [Google Scholar]
- Safford R. E., Bassingthwaighte J. B. Calcium diffusion in transient and steady states in muscle. Biophys J. 1977 Oct;20(1):113–136. doi: 10.1016/S0006-3495(77)85539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandell T., Sheppherd J. T. The effect in humans of exercise on relationship between simultaneously measured 133Xe and 24Na clearances. Scand J Clin Lab Invest. 1968;21(2):99–107. doi: 10.3109/00365516809084271. [DOI] [PubMed] [Google Scholar]
- Suenson M., Richmond D. R., Bassingthwaighte J. B. Diffusion of sucrose, sodium, and water in ventricular myocardium. Am J Physiol. 1974 Nov;227(5):1116–1123. doi: 10.1152/ajplegacy.1974.227.5.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyml K., Ellis C. G., Safranyos R. G., Fraser S., Groom A. C. Temporal and spatial distributions of red cell velocity in capillaries of resting skeletal muscle, including estimates of red cell transit times. Microvasc Res. 1981 Jul;22(1):14–31. doi: 10.1016/0026-2862(81)90108-4. [DOI] [PubMed] [Google Scholar]
- Wissig S. L., Williams M. C. Permeability of muscle capillaries to microperoxidase. J Cell Biol. 1978 Feb;76(2):341–359. doi: 10.1083/jcb.76.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudilevich D. L., Renkin E. M., Alvarez O. A., Bravo I. Fractional extraction and transcapillary exchange during continuous and instantaneous tracer administration. Circ Res. 1968 Aug;23(2):325–336. doi: 10.1161/01.res.23.2.325. [DOI] [PubMed] [Google Scholar]


