Abstract
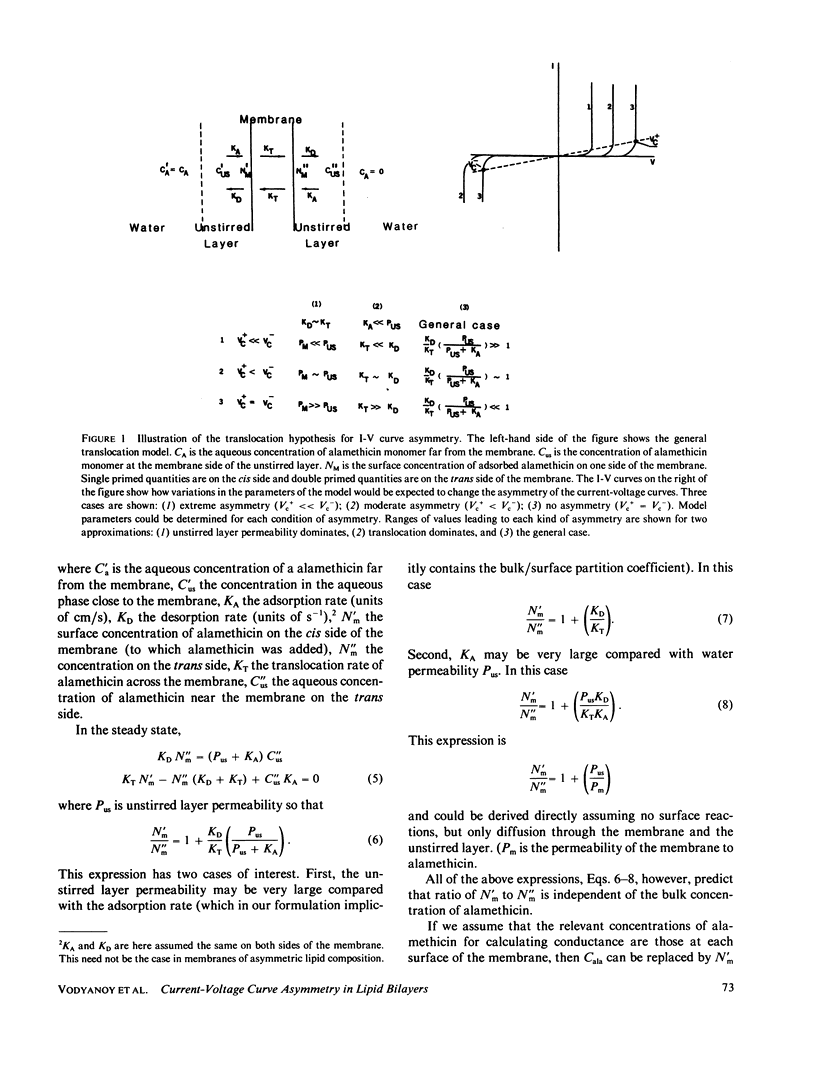
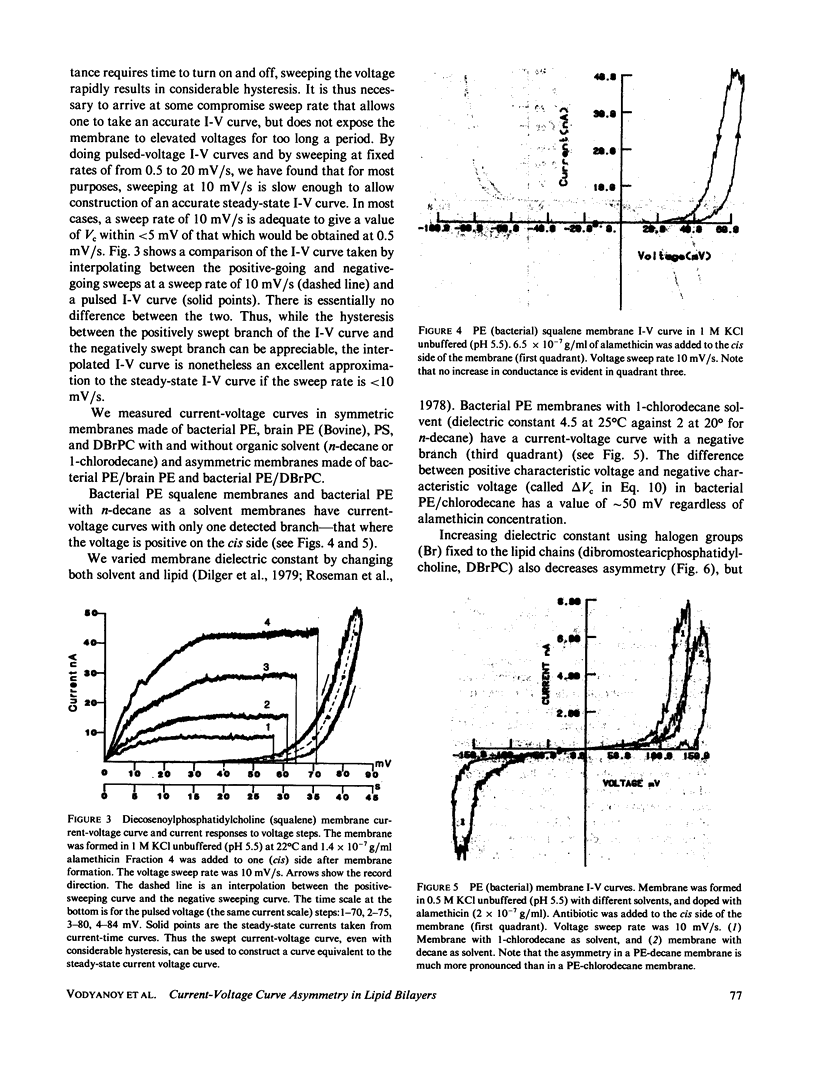
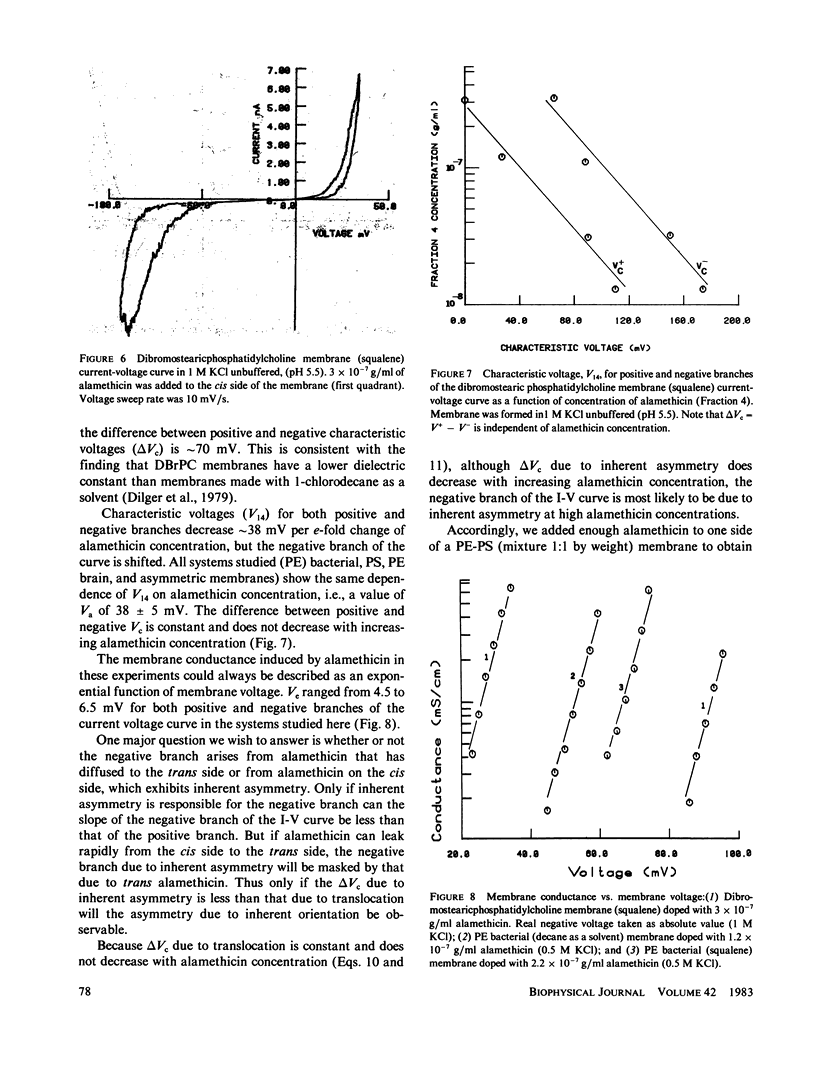
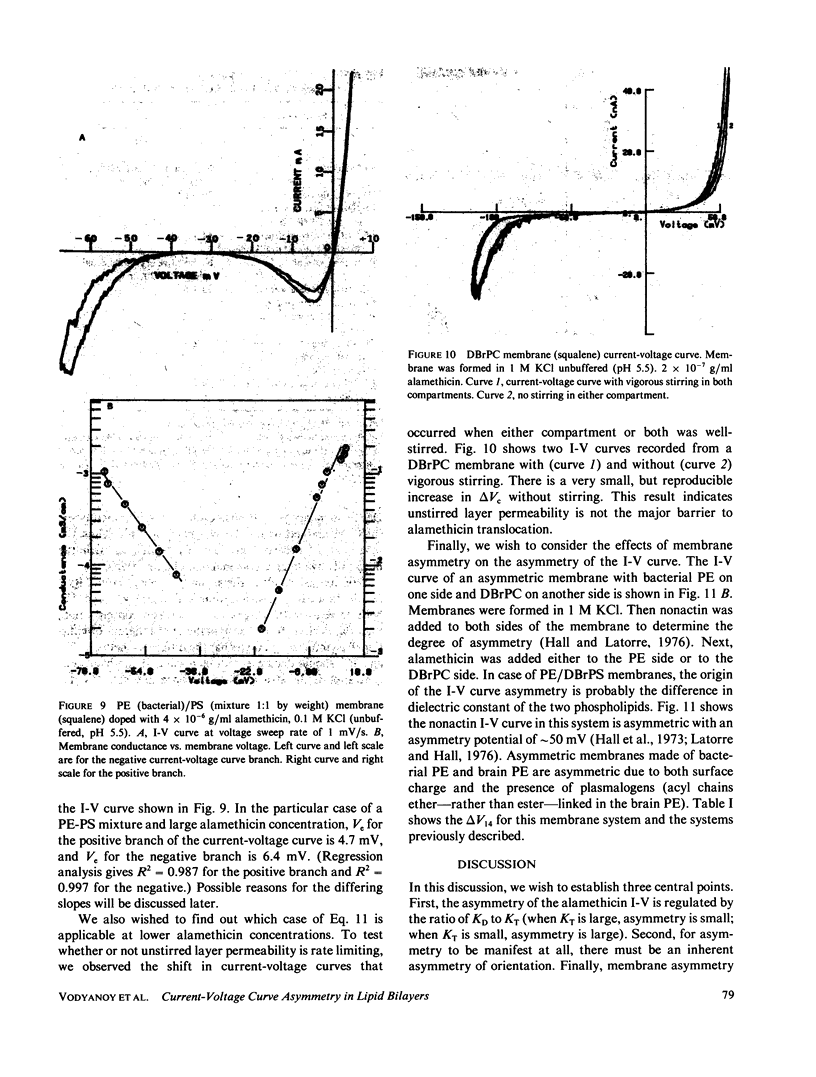
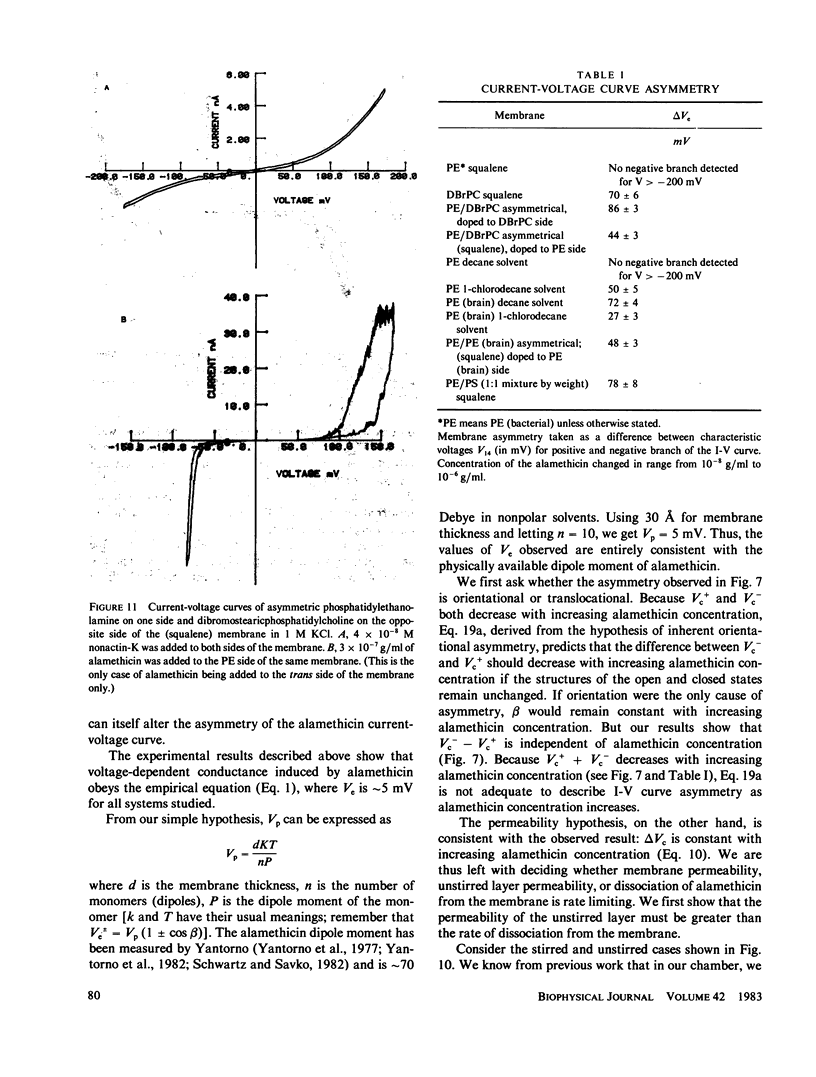
We have examined the causes of the asymmetry of the current-voltage curve induced by addition of alamethicin to one side of a black lipid membrane. We find that the alamethicin-induced current-voltage (I-V) curve has an inherent asymmetry. If it were possible to confine all alamethicin molecules to one side of the membrane, the I-V curve would exhibit a positive branch (voltage being measured with respect to the side of the membrane trans to the alamethicin addition) of steeper logarithmic slope than the negative branch and at a lower absolute value of potential. This condition is not usually realized, however, because alamethicin can leak through the membrane, so that, except at very high alamethicin concentrations and in certain kinds of membranes, the positive branch of the current-voltage curve has the same logarithmic slope as the negative branch and appears to arise from alamethicin which diffuses from the cis to the trans side of the membrane. We develop simple quantitative models for these two cases.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann G., Mueller P. A molecular model of membrane excitability. J Supramol Struct. 1974;2(5-6):538–557. doi: 10.1002/jss.400020504. [DOI] [PubMed] [Google Scholar]
- Boheim G. Statistical analysis of alamethicin channels in black lipid membranes. J Membr Biol. 1974;19(3):277–303. doi: 10.1007/BF01869983. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D., Hall J. Alamethicin channels incorporated into frog node of ranvier: calcium-induced inactivation and membrane surface charges. J Gen Physiol. 1982 Mar;79(3):411–436. doi: 10.1085/jgp.79.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger J. P., McLaughlin S. G., McIntosh T. J., Simon S. A. The dielectric constant of phospholipid bilayers and the permeability of membranes to ions. Science. 1979 Dec 7;206(4423):1196–1198. doi: 10.1126/science.228394. [DOI] [PubMed] [Google Scholar]
- Eisenberg M., Hall J. E., Mead C. A. The nature of the voltage-dependent conductance induced by alamethicin in black lipid membranes. J Membr Biol. 1973 Dec 31;14(2):143–176. doi: 10.1007/BF01868075. [DOI] [PubMed] [Google Scholar]
- Gordon L. G., Haydon D. A. Potential-dependent conductances in lipid membranes containing alamethicin. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):433–447. doi: 10.1098/rstb.1975.0021. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Cahalan M. D. Calcium-induced inactivation of alamethicin in asymmetric lipid bilayers. J Gen Physiol. 1982 Mar;79(3):387–409. doi: 10.1085/jgp.79.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. E., Latorre R. Nonactin-K+ complex as a probe for membrane asymmetry. Biophys J. 1976 Jan;16(1):99–103. doi: 10.1016/S0006-3495(76)85667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Dubischar N. Conformational changes of alamethicin induced by solvent and temperature. A 13C-NMR and circular-dichroism study. Eur J Biochem. 1975 Jun;54(2):395–409. doi: 10.1111/j.1432-1033.1975.tb04150.x. [DOI] [PubMed] [Google Scholar]
- Latorre R., Alvarez O. Voltage-dependent channels in planar lipid bilayer membranes. Physiol Rev. 1981 Jan;61(1):77–150. doi: 10.1152/physrev.1981.61.1.77. [DOI] [PubMed] [Google Scholar]
- Latorre R., Hall J. E. Dipole potential measurements in asymmetric membranes. Nature. 1976 Nov 25;264(5584):361–363. doi: 10.1038/264361a0. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potentials induced in biomolecular lipid membranes. Nature. 1968 Feb 24;217(5130):713–719. doi: 10.1038/217713a0. [DOI] [PubMed] [Google Scholar]
- Muller R. U., Finkelstein A. Voltage-dependent conductance induced in thin lipid membranes by monazomycin. J Gen Physiol. 1972 Sep;60(3):263–284. doi: 10.1085/jgp.60.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumcke B., Läuger P. Nonlinear electrical effects in lipid bilayer membranes. II. Integration of the generalized Nernst-Planck equations. Biophys J. 1969 Sep;9(9):1160–1170. doi: 10.1016/S0006-3495(69)86443-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusser F. Biosynthesis of antibiotic U-22,324, a cyclic polypeptide. J Biol Chem. 1967 Jan 25;242(2):243–247. [PubMed] [Google Scholar]
- Roy G. Properties of the conductance induced in lecithin bilayer membranes by alamethicin. J Membr Biol. 1975 Oct 16;24(1):71–85. doi: 10.1007/BF01868616. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Boheim G. Alamethicin-induced single channel conductance fluctuations in biological membranes. Nature. 1979 Nov 15;282(5736):336–339. doi: 10.1038/282336a0. [DOI] [PubMed] [Google Scholar]
- Schindler H., Feher G. Branched bimolecular lipid membranes. Biophys J. 1976 Sep;16(9):1109–1113. doi: 10.1016/S0006-3495(76)85759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G., Savko P. Structural and dipolar properties of the voltage-dependent pore former alamethicin in octanol/dioxane. Biophys J. 1982 Aug;39(2):211–219. doi: 10.1016/S0006-3495(82)84510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantorno R., Takashima S., Mueller P. Dipole moment of alamethicin as related to voltage-dependent conductance in lipid bilayers. Biophys J. 1982 May;38(2):105–110. doi: 10.1016/S0006-3495(82)84536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


