Abstract
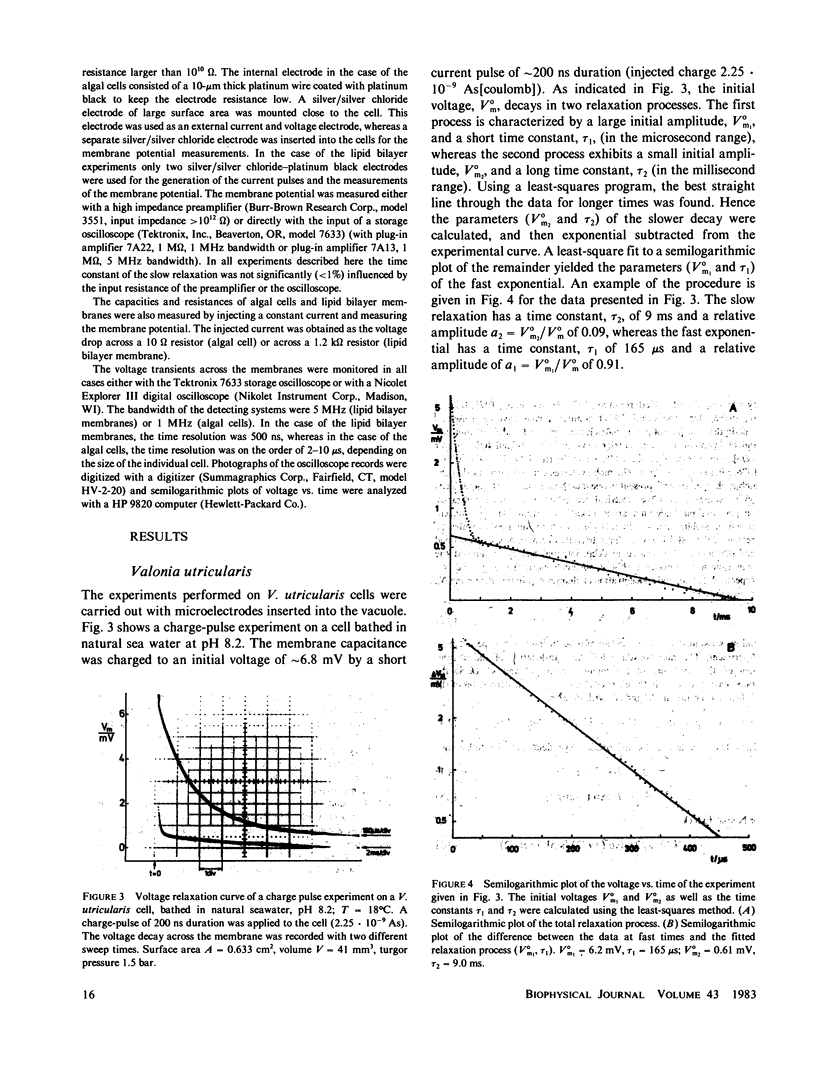
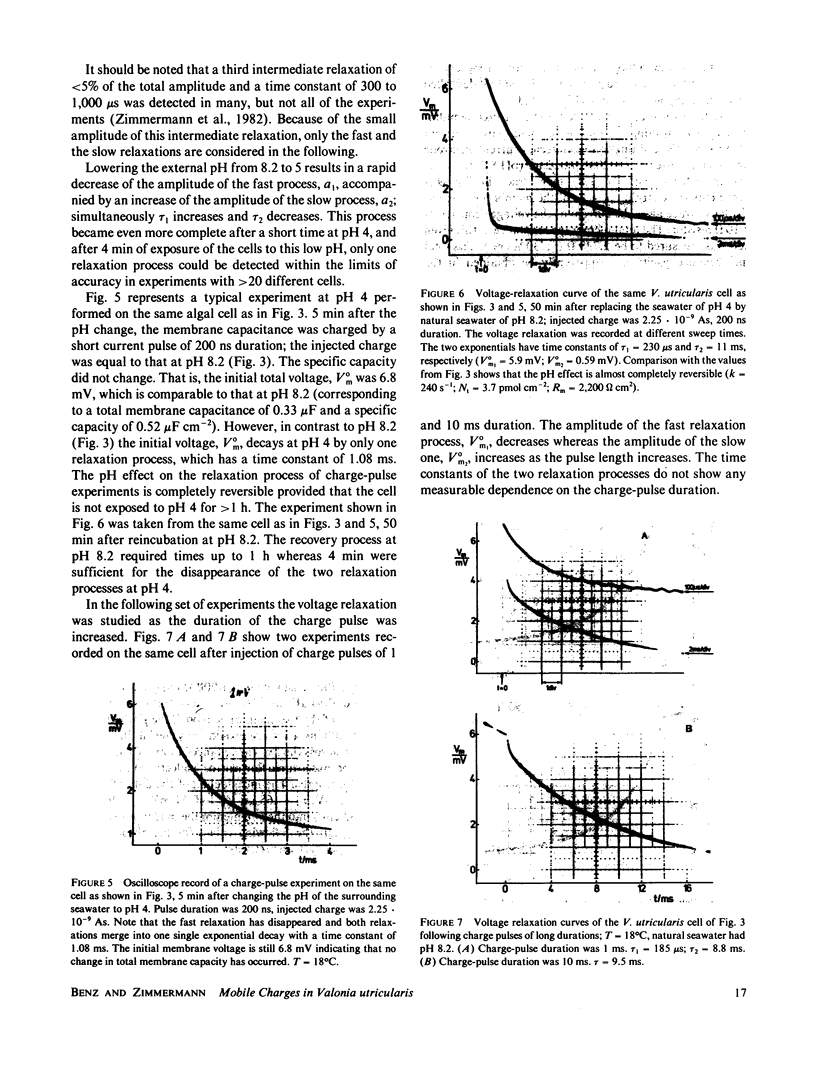
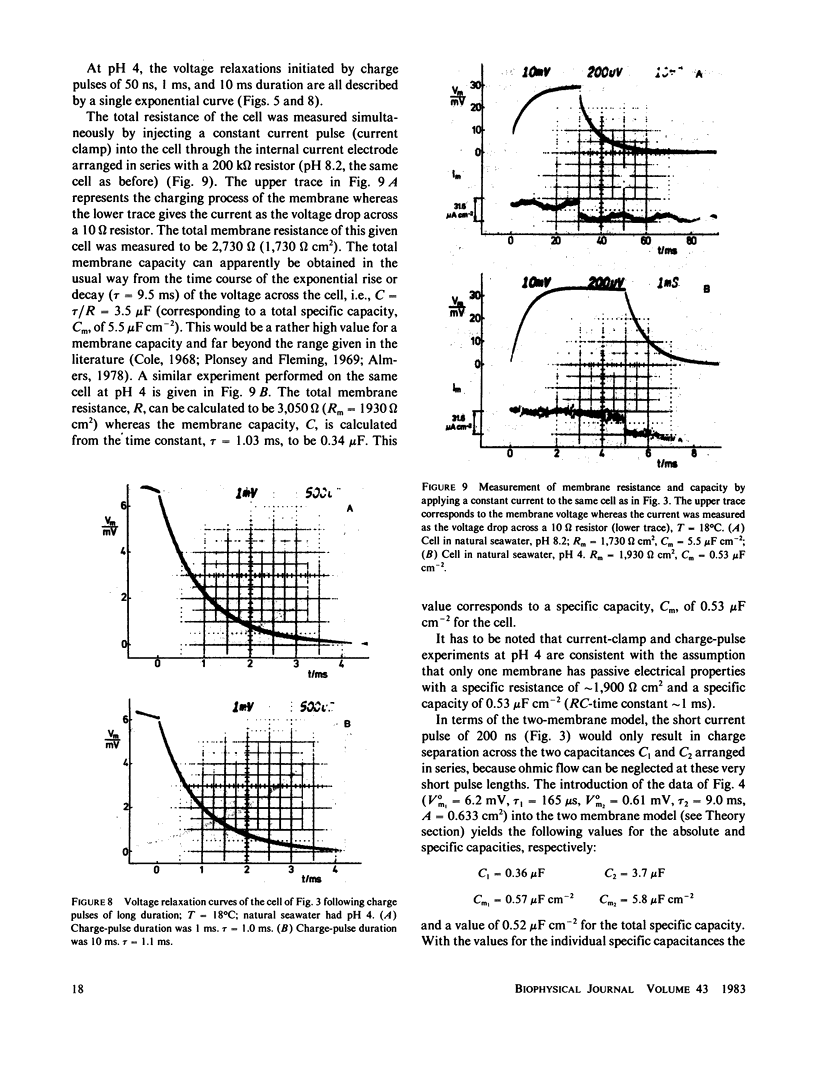
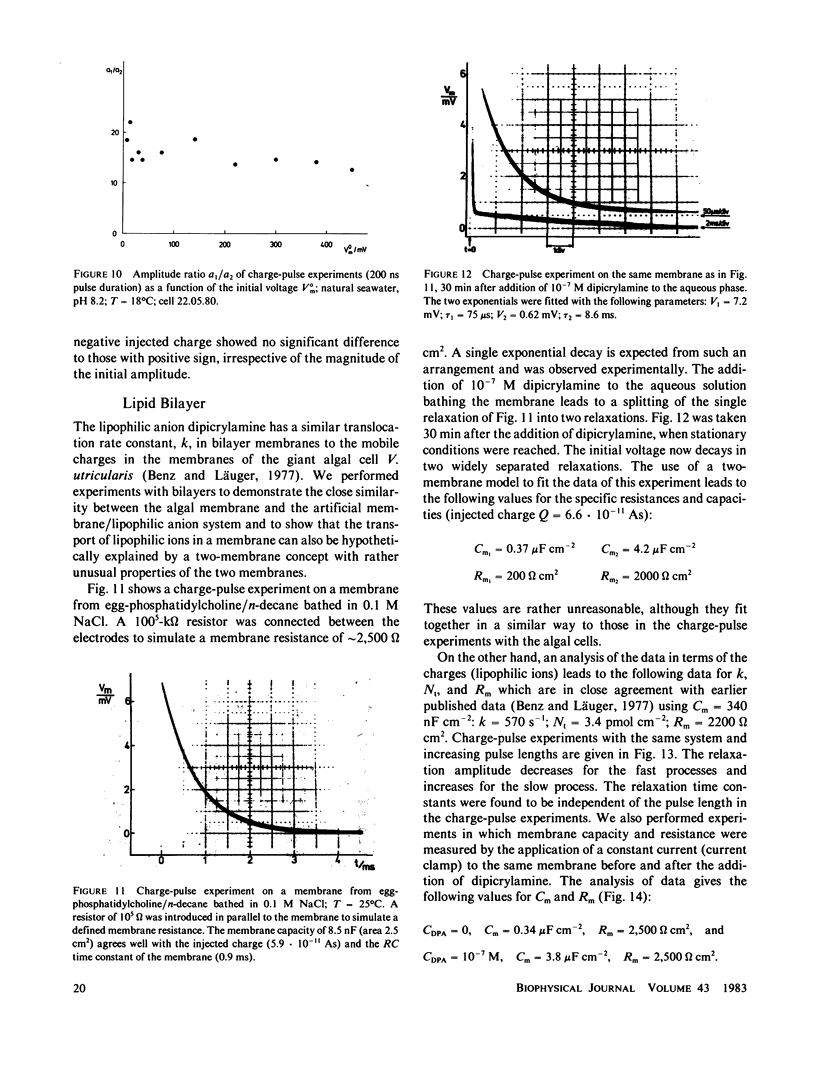
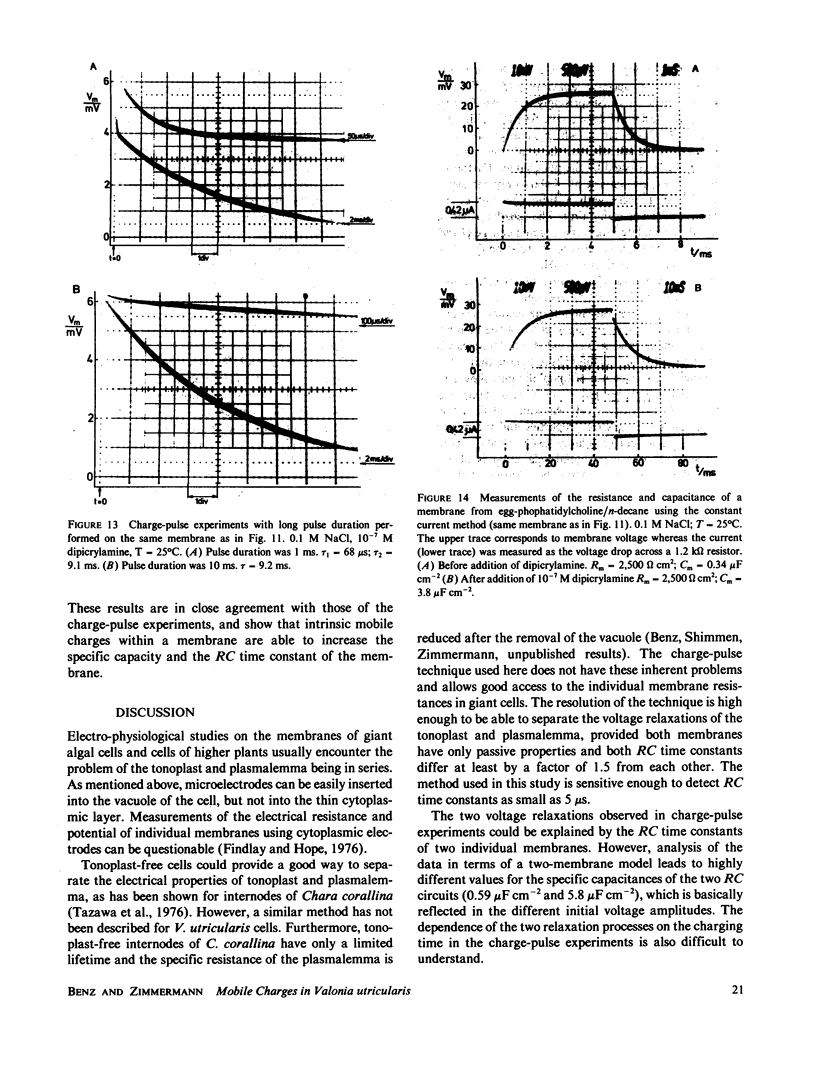
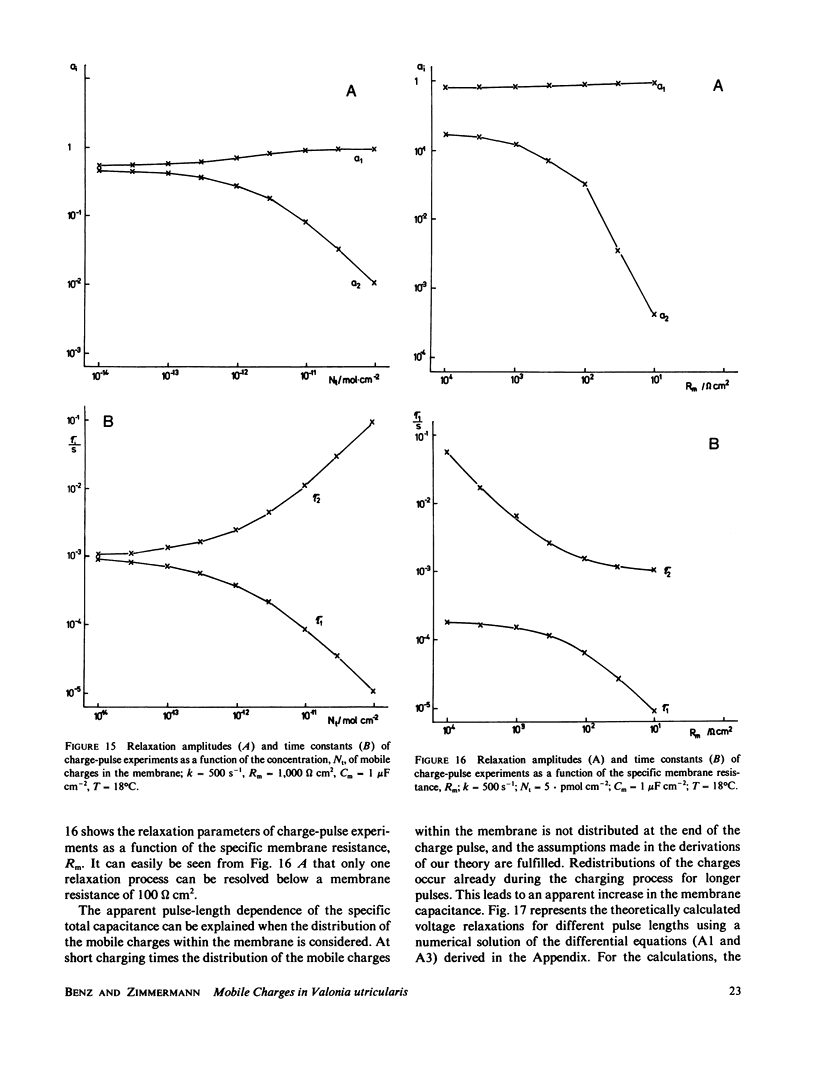
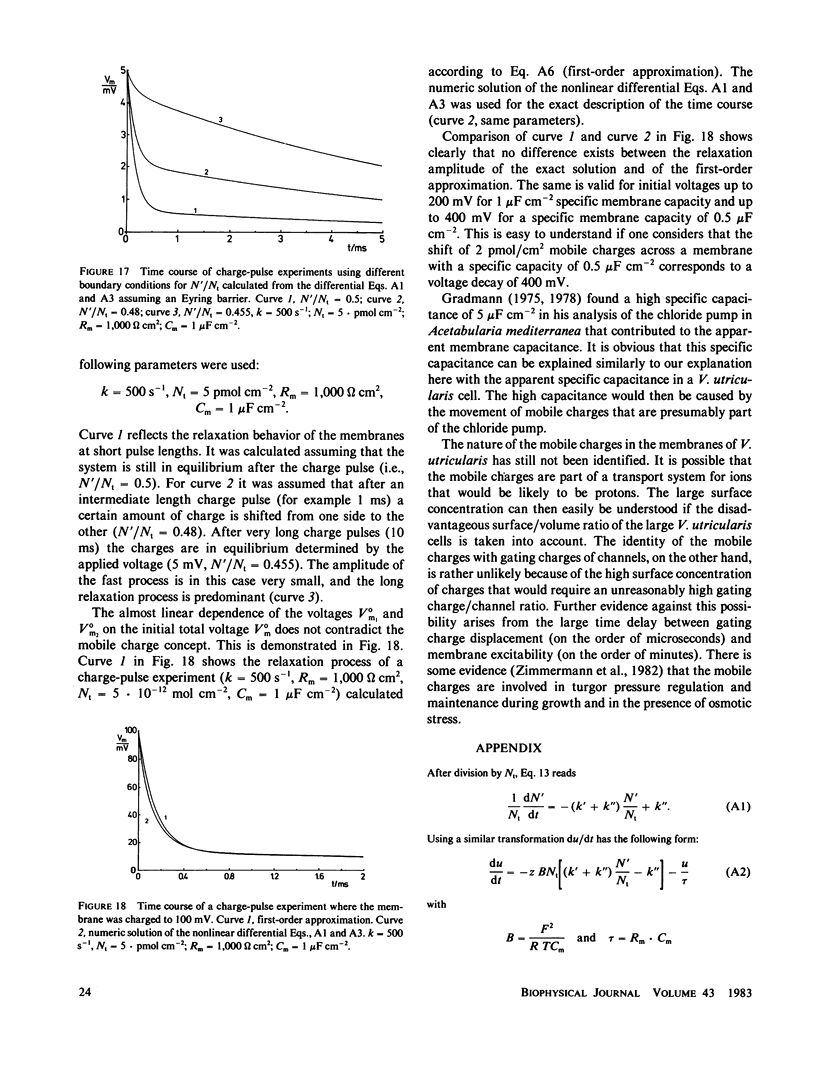
Charge-pulse relaxation studies were performed on cells of the giant marine alga Valonia utricularis with microelectrodes inserted into the vacuole. If the cell was charged by short pulses of 200 ns duration, the decay of the initial membrane voltage could be described by two relaxation processes at normal pH (8.2). The fast exponential relaxation had a time constant of approximately 100 microseconds whereas the the time constant of the slow relaxation ranged between 2 and 15 ms. The ratio of the two amplitudes varied between 10 and 20 and was found to be independent of the initial voltage, up to 400 mV. In contrast to the time constants, the amplitude ratio was a function of the duration of the charge pulse. As the pulse length was increased to 10 ms, the fast relaxation disappeared. A change in pH of the natural sea water from 8.2 to 4 resulted in the disappearance of both exponential processes and the appearance of one single exponential with a 1-ms time constant over the whole pulse-length range. The analysis of the data in terms of a two-membrane model leads to unusual values and a pH-dependence of the specific capacitances (0.6 and 6 microF cm-2) of the two membranes, which can be treated as two serial circuits of a capacitor and a resistor in parallel. The charge-pulse and the current-clamp data are consistent with the assumption that the cell membrane of V. utricularis contains mobile charges with a total surface concentration of approximately 4 pmol cm-2. These charges cross the membrane barrier with a translocation rate constant around 500 s-1 and become neutralized at low pH. From our experimental results it cannot be completely excluded that the tonoplast has also a high specific resistance. But in this case it has to be assumed that the tonoplast and plasmalemma have very similar electrical properties and contain both mobile charges, so that the two membranes appear as a single membrane. Experiments on artificial lipid bilayer membranes in the presence of the lipophilic ion dipicrylamine, support our mobile charge concept for the cell membrane of V. utricularis.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W. Gating currents and charge movements in excitable membranes. Rev Physiol Biochem Pharmacol. 1978;82:96–190. doi: 10.1007/BFb0030498. [DOI] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973 Apr 13;242(5398):459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- Benz R. Alkali ion transport through lipid bilayer membranes mediated by enniatin A and B and beauvericin. J Membr Biol. 1978 Nov 8;43(4):367–394. doi: 10.1007/BF01871697. [DOI] [PubMed] [Google Scholar]
- Benz R., Beckers F., Zimmermann U. Reversible electrical breakdown of lipid bilayer membranes: a charge-pulse relaxation study. J Membr Biol. 1979 Jul 16;48(2):181–204. doi: 10.1007/BF01872858. [DOI] [PubMed] [Google Scholar]
- Benz R., Conti F. Structure of the squid axon membrane as derived from charge-pulse relaxation studies in the presence of absorbed lipophilic ions. J Membr Biol. 1981 Apr 15;59(2):91–104. doi: 10.1007/BF01875707. [DOI] [PubMed] [Google Scholar]
- Benz R., Cros D. Influence of sterols on ion transport through lipid bilayer membranes. Biochim Biophys Acta. 1978 Jan 19;506(2):265–280. doi: 10.1016/0005-2736(78)90397-8. [DOI] [PubMed] [Google Scholar]
- Benz R., Gisin B. F. Influence of membrane structure on ion transport through lipid bilayer membranes. J Membr Biol. 1978 Jun 9;40(4):293–314. doi: 10.1007/BF01874161. [DOI] [PubMed] [Google Scholar]
- Benz R., Gisin B. F., Ting-Beall H. P., Tosteson D. C., Läuger P. Mechanism of ion transport through lipid bilayer-membranes mediated by peptide cyclo-(D-Val-L-Pro-L-Val-D-Pro). Biochim Biophys Acta. 1976 Dec 14;455(3):665–684. doi: 10.1016/0005-2736(76)90040-7. [DOI] [PubMed] [Google Scholar]
- Benz R., Janko K. Voltage-induce capacitance relaxation of lipid bilayer membranes. Effects of membrane composition. Biochim Biophys Acta. 1976 Dec 14;455(3):721–738. doi: 10.1016/0005-2736(76)90043-2. [DOI] [PubMed] [Google Scholar]
- Benz R., Läuger P., Janko K. Transport kinetics of hydrophobic ions in lipid bilayer membranes. Charge-pulse relaxation studies. Biochim Biophys Acta. 1976 Dec 14;455(3):701–720. doi: 10.1016/0005-2736(76)90042-0. [DOI] [PubMed] [Google Scholar]
- Benz R., Läuger P. Kinetic analysis of carrier-mediated ion transport by the charge-pulse technique. J Membr Biol. 1976 Jun 9;27(1-2):171–191. doi: 10.1007/BF01869135. [DOI] [PubMed] [Google Scholar]
- Benz R., Läuger P. Transport kinetics of dipicrylamine through lipid bilayer membranes. Effects of membrane structure. Biochim Biophys Acta. 1977 Jul 14;468(2):245–258. doi: 10.1016/0005-2736(77)90118-3. [DOI] [PubMed] [Google Scholar]
- Benz R., McLaughlin S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J. 1983 Mar;41(3):381–398. doi: 10.1016/S0006-3495(83)84449-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Stark G., Janko K., Läuger P. Valinomycin-mediated ion transport through neutral lipid membranes: influence of hydrocarbon chain length and temperature. J Membr Biol. 1973;14(4):339–364. doi: 10.1007/BF01868084. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Schneider M. F., Rakowski R. F., Adrian R. H. Charge movements in skeletal muscle. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):501–505. doi: 10.1098/rstb.1975.0026. [DOI] [PubMed] [Google Scholar]
- Davis R. F. Electrical Properties of the Plasmalemma and Tonoplast in Valonia ventricosa. Plant Physiol. 1981 Apr;67(4):825–831. doi: 10.1104/pp.67.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H. Amine transport at the plasmalemma of Riccia fluitans. Biochim Biophys Acta. 1980 Oct 16;602(1):181–195. doi: 10.1016/0005-2736(80)90300-4. [DOI] [PubMed] [Google Scholar]
- Felle H., Bentrup F. W. A study of the primary effect of the uncoupler carbonyl cyanide m-chlorophenylhydrazone on membrane potential and conductance in Riccia fluitans. Biochim Biophys Acta. 1977 Jan 4;464(1):179–187. doi: 10.1016/0005-2736(77)90380-7. [DOI] [PubMed] [Google Scholar]
- Gradmann D. Analog circuit of the Acetabularia membrane. J Membr Biol. 1975 Dec 4;25(1-2):183–208. doi: 10.1007/BF01868574. [DOI] [PubMed] [Google Scholar]
- Kagawa Y. Reconstitution of the energy transformer, gate and channel subunit reassembly, crystalline ATPase and ATP synthesis. Biochim Biophys Acta. 1978 Sep 21;505(1):45–93. doi: 10.1016/0304-4173(78)90008-3. [DOI] [PubMed] [Google Scholar]
- Komor E., Tanner W. The determination of the membrane ptoential of Chlorella vulgaris. Evidence for electrogenic sugar transport. Eur J Biochem. 1976 Nov 1;70(1):197–204. doi: 10.1111/j.1432-1033.1976.tb10970.x. [DOI] [PubMed] [Google Scholar]
- Konings W. N. Active transport of solutes in bacterial membrane vesicles. Adv Microb Physiol. 1977;15:175–251. doi: 10.1016/s0065-2911(08)60317-3. [DOI] [PubMed] [Google Scholar]
- Lainson R., Field C. D. Electrical properties of Valonia ventricosa. J Membr Biol. 1976 Oct 20;29(1-2):81–94. doi: 10.1007/BF01868953. [DOI] [PubMed] [Google Scholar]
- Nonner W., Rojas E., Stämpfli R. Gating currents in the node of Ranvier: voltage and time dependence. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):483–492. doi: 10.1098/rstb.1975.0024. [DOI] [PubMed] [Google Scholar]
- PAULY H. Electrical properties of the cytoplasmic membrane and the cytoplasm of bacteria and of protoplasts. Ire Trans Biomed Electron. 1962 Apr;BME-9:93–95. doi: 10.1109/tbmel.1962.4322970. [DOI] [PubMed] [Google Scholar]
- Rousselet A., Colbeau A., Vignais P. M., Devaux P. F. Study of the transverse diffusion of spin-labeled phospholipids in biological membranes. II. Inner mitochondrial membrane of rat liver: use of phosphatidylcholine exchange protein. Biochim Biophys Acta. 1976 Mar 19;426(3):372–384. doi: 10.1016/0005-2736(76)90383-7. [DOI] [PubMed] [Google Scholar]
- Rousselet A., Guthmann C., Matricon J., Bienvenue A., Devaux P. F. Study of the transverse diffusion of spin labeled phospholipids in biological membranes. I. Human red bloods cells. Biochim Biophys Acta. 1976 Mar 19;426(3):357–371. doi: 10.1016/0005-2736(76)90382-5. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Depolarization of the plasma membrane of Neurospora during active transport of glucose: evidence for a proton-dependent cotransport system. Proc Natl Acad Sci U S A. 1974 May;71(5):1935–1939. doi: 10.1073/pnas.71.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann U., Steudle E. The pressure-dependence of the hydraulic conductivity, the membrane resistance and membrane potential during turgor pressure regulation in Valonia utricularis. J Membr Biol. 1974;16(4):331–352. doi: 10.1007/BF01872422. [DOI] [PubMed] [Google Scholar]


