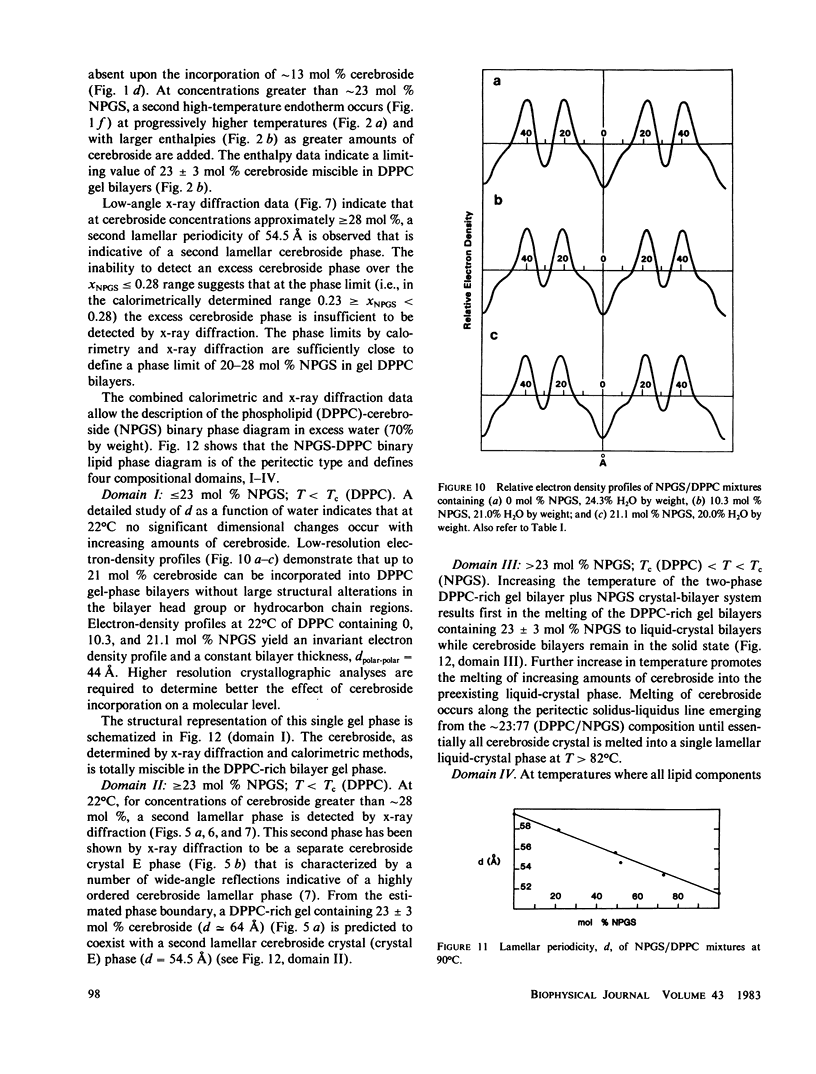
Abstract
Differential scanning calorimetry (DSC) and x-ray diffraction have been used to study the interaction of hydrated N-palmitoylgalactosylsphingosine (NPGS) and dipalmitoylphosphatidylcholine (DPPC). For mixtures containing less than 23 mol% NPGS, complete miscibility of NPGS into hydrated DPPC bilayers is observed in both the bilayer gel and liquid-crystal phases. X-ray diffraction data demonstrate insignificant differences in the DPPC-bilayer gel-phase parameters on incorporation of up to 23 mol% NPGS. At greater than 23 mol% NPGS, additional high-temperature transitions occur, indicating phase separation of cerebroside. For these cerebroside concentrations, at 20 degrees C, x-ray diffraction shows two lamellar phases, hydrated DPPC-NPGS gel bilayers (d = 64 A) containing 23 mol% NPGS, and NPGS "crystal" bilayers (d = 55 A). On heating to temperatures greater than 45 degrees C, the mixed DPPC-NPGS bilayer phase undergoes chain melting, and on further increasing the temperature progressively more NPGS is incorporated into the liquid-crystal DPPC-NPGS bilayer phase. At temperatures greater than 82 degrees C (the transition temperature of hydrated NPGS), complete lipid miscibility is observed at all DPPC/NPGS molar ratios.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bologa-Sandru L., Zalc B., Herschkowitz N., Baumann N. Oligodendrocytes of jimpy mice express galactosylceramide: an immunofluorescence study on brain sections and dissociated brain cell cultures. Brain Res. 1981 Nov 30;225(2):425–430. doi: 10.1016/0006-8993(81)90848-9. [DOI] [PubMed] [Google Scholar]
- Bunow M. R., Levin I. W. Molecular conformations of cerebrosides in bilayers determined by Raman spectroscopy. Biophys J. 1980 Dec;32(3):1007–1021. doi: 10.1016/S0006-3495(80)85032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunow M. R. Two gel states of cerebrosides. Calorimetric and Raman spectroscopic evidence. Biochim Biophys Acta. 1979 Sep 28;574(3):542–546. doi: 10.1016/0005-2760(79)90250-9. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Cherry R. J., Chapman D. Physical properties of lecithin-cerebroside bilayers. Biochim Biophys Acta. 1971 Oct 12;249(1):301–317. doi: 10.1016/0005-2736(71)90108-8. [DOI] [PubMed] [Google Scholar]
- Correa-Freire M. C., Freire E., Barenholz Y., Biltonen R. L., Thompson T. E. Thermotropic behavior of monoglucocerebroside--dipalmitoylphosphatidylcholine multilamellar liposomes. Biochemistry. 1979 Feb 6;18(3):442–445. doi: 10.1021/bi00570a008. [DOI] [PubMed] [Google Scholar]
- Curatolo W. Thermal behavior of fractionated and unfractionated bovine brain cerebrosides. Biochemistry. 1982 Apr 13;21(8):1761–1764. doi: 10.1021/bi00537a010. [DOI] [PubMed] [Google Scholar]
- Fernandez-Bermudez S., Loboda-Cacković J., Cacković H., Hosemann R. Structure of cerebrosides I. Phrenosine at 23 degrees C and 66 degrees C. Z Naturforsch C. 1977 May-Jun;32(5-6):362–374. doi: 10.1515/znc-1977-5-608. [DOI] [PubMed] [Google Scholar]
- Freire E., Bach D., Correa-Freire M., Miller I., Barenholz Y. Calorimetric investigation of the complex phase behavior of glucocerebroside dispersions. Biochemistry. 1980 Aug 5;19(16):3662–3665. doi: 10.1021/bi00557a004. [DOI] [PubMed] [Google Scholar]
- Hosemann R., Loboda-Cacković J., Cacković H., Fernandez-Bermúdez S., Baltá-Calleja F. J. Structure of cerebrosides. II. Small angle X-ray diffraction study of cerasine. Z Naturforsch C. 1979 Dec;34(12):1121–1124. doi: 10.1515/znc-1979-1206. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Jenkinson T. J., Kamat V. B., Chapman D. Physical studies of myelin. I. Thermal analysis. Biochim Biophys Acta. 1968 Sep 2;164(1):101–109. doi: 10.1016/0005-2760(68)90076-3. [DOI] [PubMed] [Google Scholar]
- Larsson D., Karlsson D. A. Molecular arrangements in glycosphingolipids. Chem Phys Lipids. 1972 Mar;8(2):152–179. doi: 10.1016/0009-3084(72)90027-8. [DOI] [PubMed] [Google Scholar]
- Lee R. E., Worthington C. R., Glew R. H. The bilayer nature of deposits occurring in Gaucher's disease. Arch Biochem Biophys. 1973 Nov;159(1):259–266. doi: 10.1016/0003-9861(73)90452-9. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Bailey A. I., Wilkins M. H. Multilayers of phospholipid bimolecular leaflets. Nature. 1968 Nov 9;220(5167):577–578. doi: 10.1038/220577a0. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J. The effect of cholesterol on the structure of phosphatidylcholine bilayers. Biochim Biophys Acta. 1978 Oct 19;513(1):43–58. doi: 10.1016/0005-2736(78)90110-4. [DOI] [PubMed] [Google Scholar]
- Reiss-Husson F. Structure des phases liquide-cristallines de différents phospholipides, monoglycérides, sphingolipides, anhydres ou en présence d'eau. J Mol Biol. 1967 May 14;25(3):363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- Rumsby M. G. Organization and structure in central-nerve myelin. Biochem Soc Trans. 1978;6(2):448–462. doi: 10.1042/bst0060448. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Atkinson D., Small D. M., Skarjune R. P., Oldfield E., Shipley G. G. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 1981 Oct 13;20(21):5957–5966. doi: 10.1021/bi00524a006. [DOI] [PubMed] [Google Scholar]
- Skarjune R., Oldfield E. Physical studies of cell surface and cell membrane structure. Deuterium nuclear magnetic resonance investigation of deuterium-labelled N-hexadeconoylgalactosylceramides (cerebrosides). Biochim Biophys Acta. 1979 Sep 21;556(2):208–218. doi: 10.1016/0005-2736(79)90043-9. [DOI] [PubMed] [Google Scholar]
- Skarjune R., Oldfield E. Physical studies of cell surface and cell membrane structure. Deuterium nuclear magnetic resonance studies of N-palmitoylglucosylceramide (cerebroside) head group structure. Biochemistry. 1982 Jun 22;21(13):3154–3160. doi: 10.1021/bi00256a019. [DOI] [PubMed] [Google Scholar]
- Tardieu A., Luzzati V., Reman F. C. Structure and polymorphism of the hydrocarbon chains of lipids: a study of lecithin-water phases. J Mol Biol. 1973 Apr 25;75(4):711–733. doi: 10.1016/0022-2836(73)90303-3. [DOI] [PubMed] [Google Scholar]