Abstract
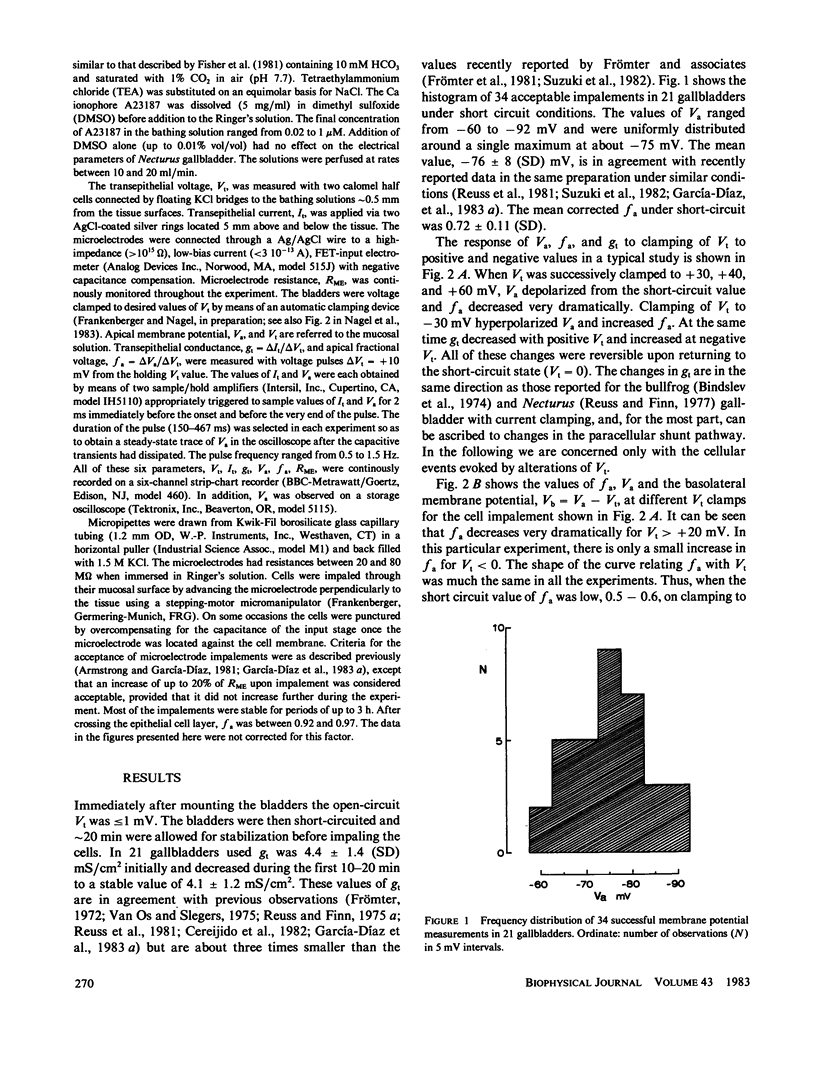
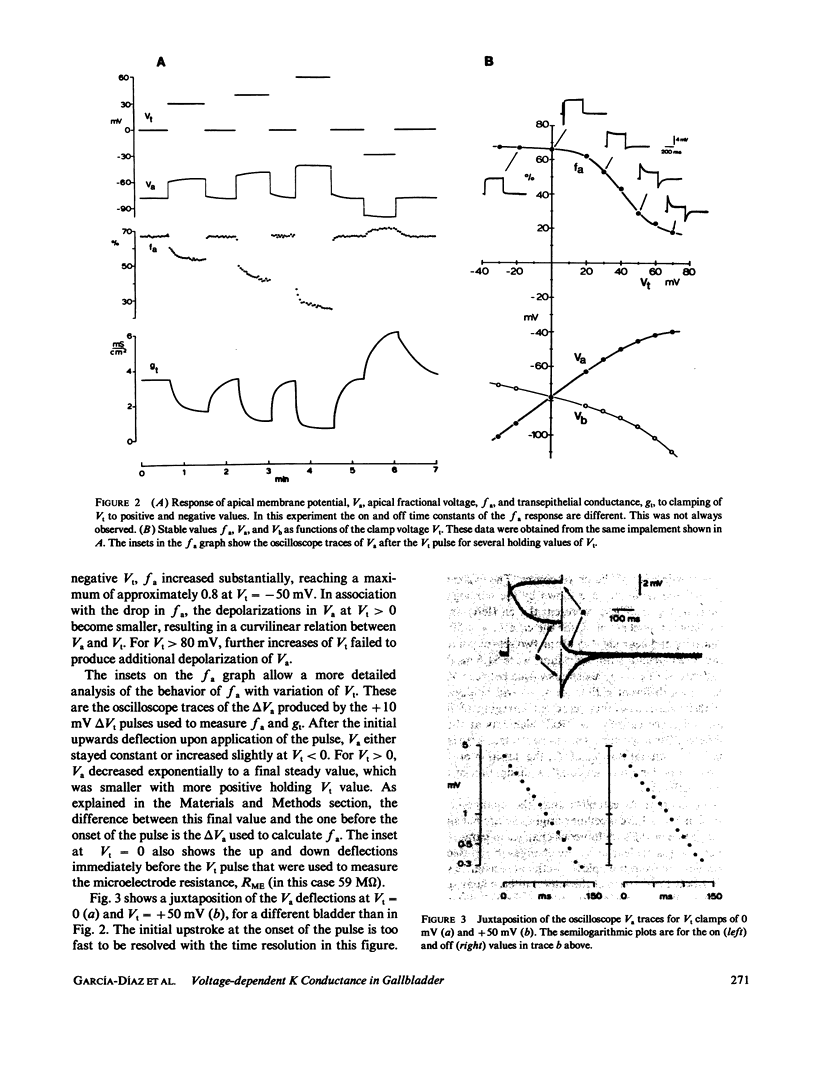
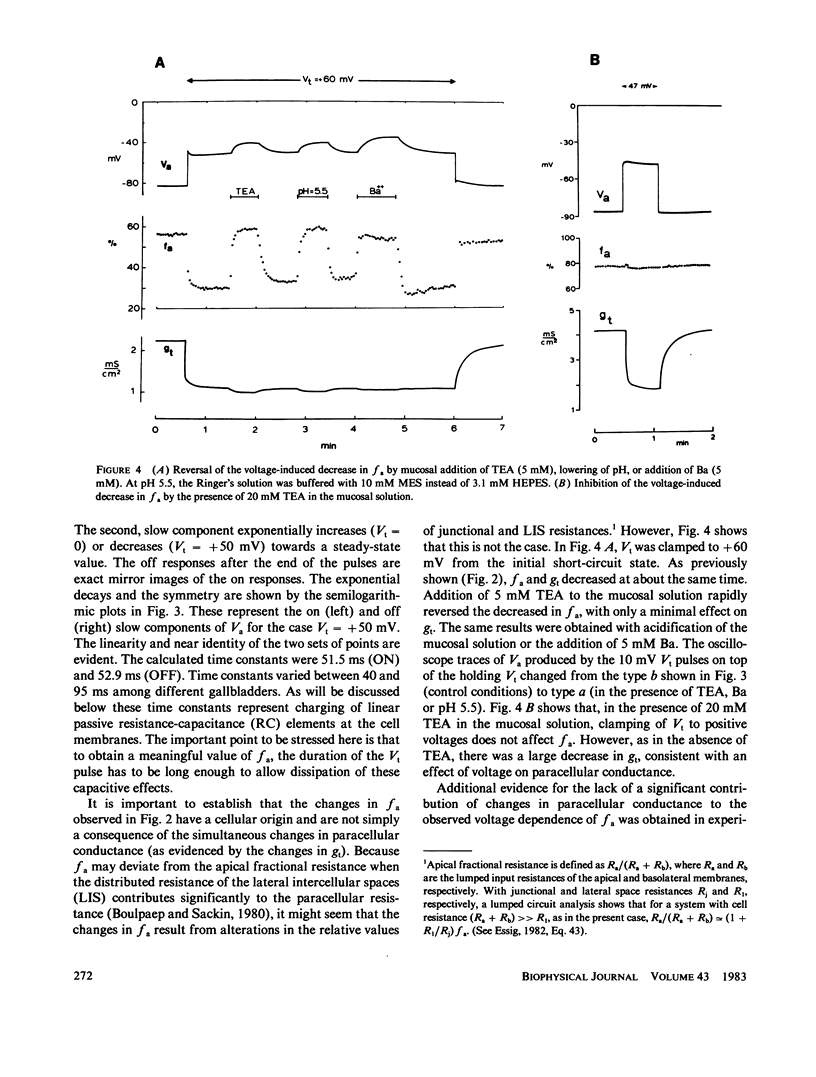
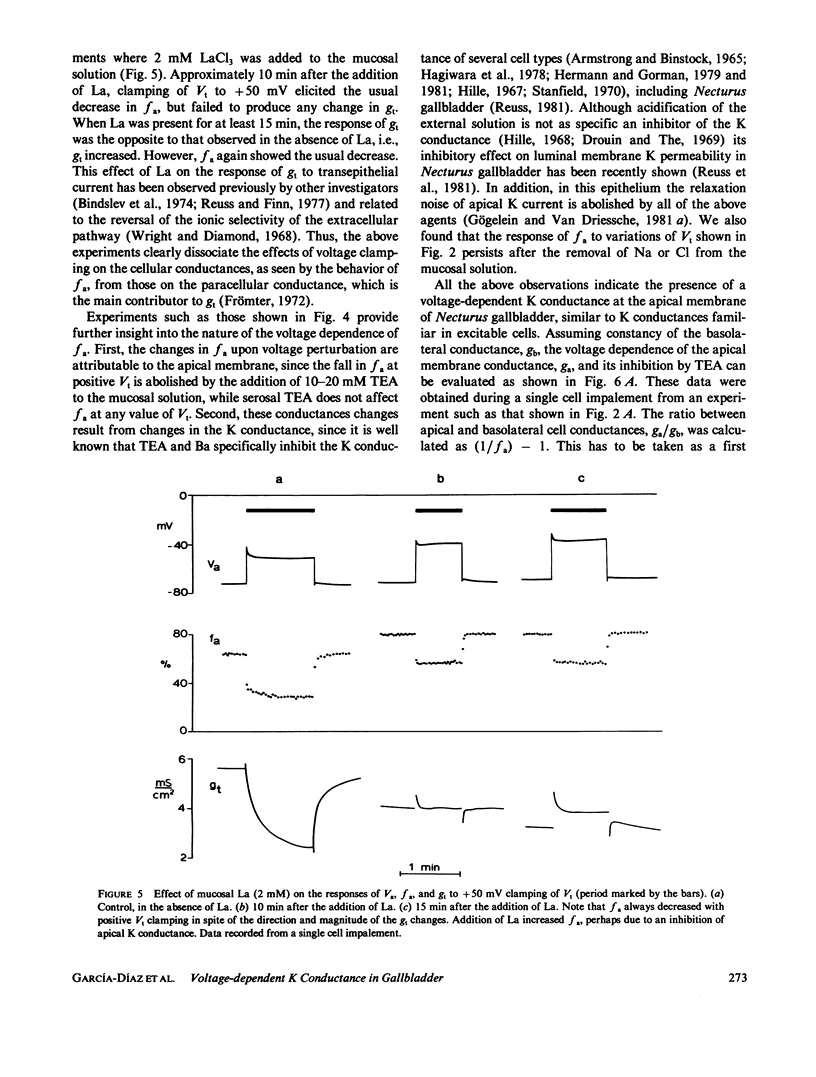
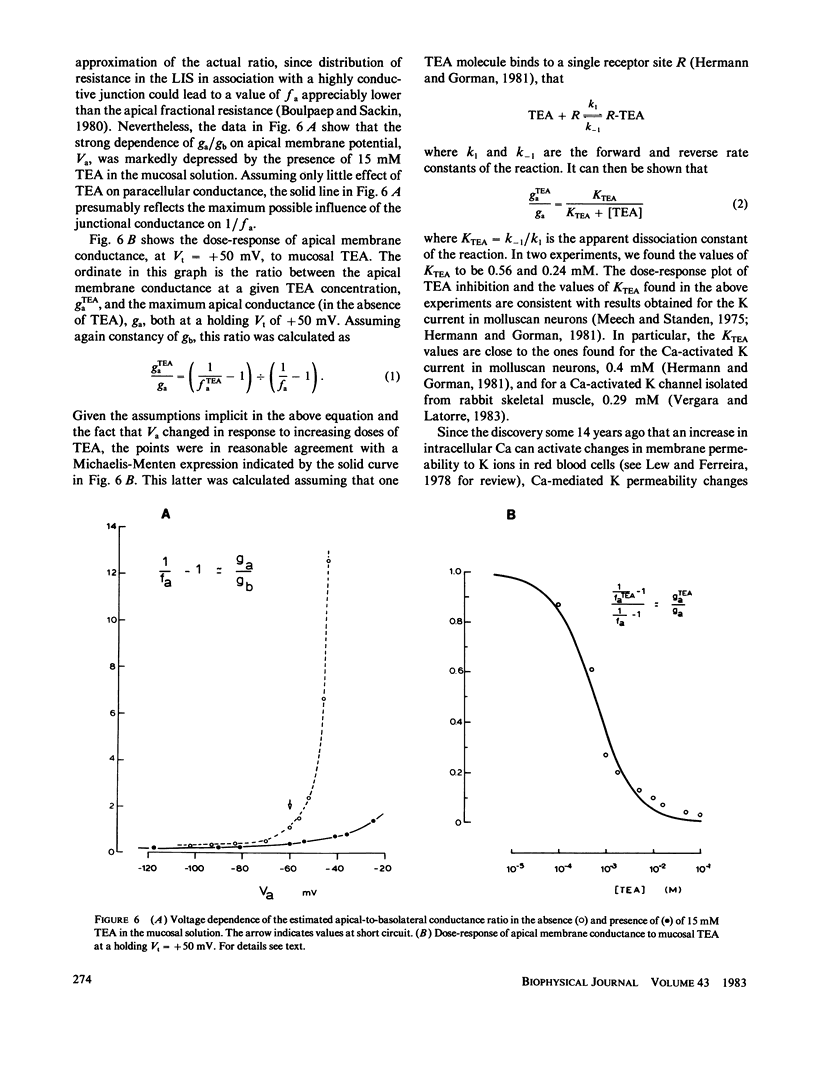
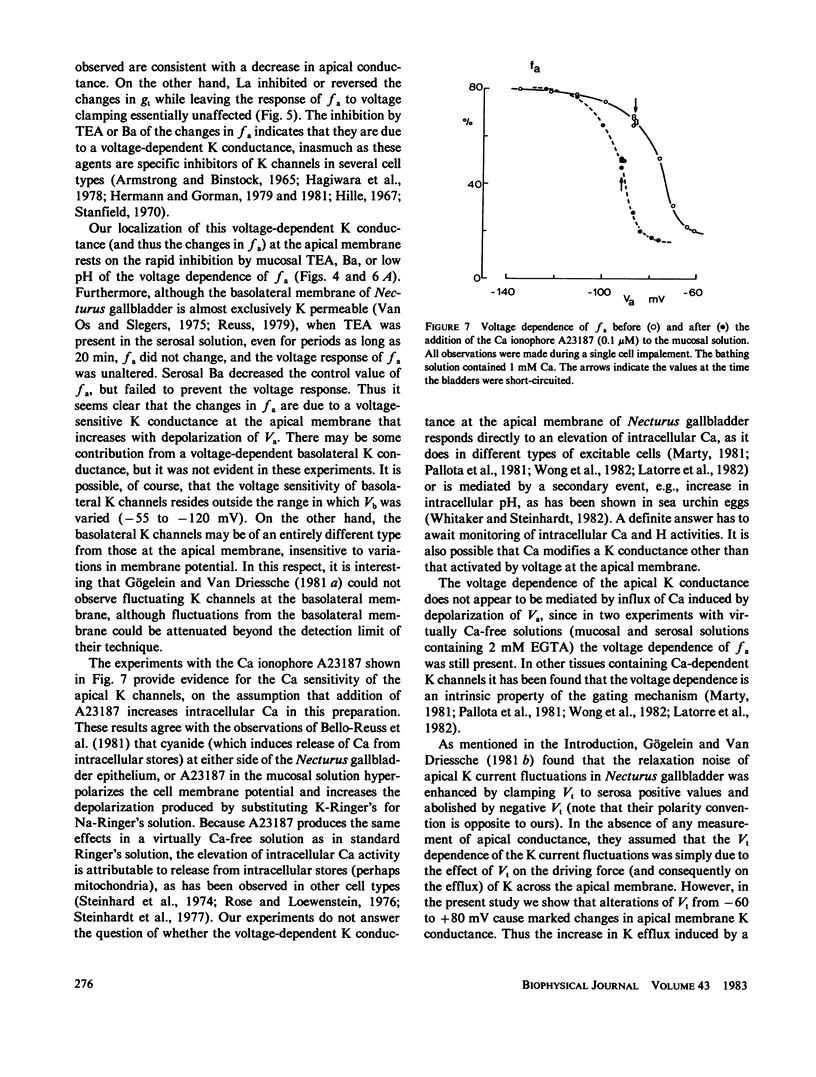
The epithelial and cellular effects of clamping the transepithelial potential (Vt, mucosa reference) have been investigated in the Necturus gallbladder. Following initial equilibration at short circuit, tissue conductance gt was 4.1 +/- 1.2 (SD) mS/cm2, the apical potential Va was -76 +/- 8 mV, and the apical fractional voltage on brief voltage perturbation (fa = delta Va/delta Vt, reflecting the ratio of apical membrane to transcellular resistance) was 0.72 +/- 0.11 (21 gallbladders, 34 impalements). On clamping Vt at positive values, Va depolarized and fa decreased; at the same time gt decreased. Clamping Vt at negative values produced converse effects. All of the above changes were related directly to the magnitude of the clamping potential Vt and were reversed on return to the short circuit state. Effects of Vt on fa are not due to changes in the extracellular pathway resistances (which, however, contribute to gt). Furthermore, the effects of Vt on fa were abolished by the mucosal application of TEA or Ba, or acidification of the mucosal solution. Thus, these experiments disclose the presence of a voltage-dependent apical K conductance that increases with apical membrane depolarization. The calculated dose-response curve of TEA inhibition of apical conductance and the values of the apparent dissociation constant were in good agreement with those found for K channels in excitable tissues. Mucosal application of the Ca ionophore A23187 shifted the voltage dependence curve of fa to more negative values of Va without altering its shape. The effect of A23187 suggests a possible role of intracellular Ca in the modulation of the apical K channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Reuss E., Grady T. P., Reuss L. Mechanism of the effect of cyanide on cell membrane potentials in Necturus gall-bladder epithelium. J Physiol. 1981 May;314:343–357. doi: 10.1113/jphysiol.1981.sp013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindslev N., Tormey J. M., Wright E. M. The effects of electrical and osmotic gradients on lateral intercellular spaces and membrane conductance in a low resistance epithelium. J Membr Biol. 1974;19(4):357–380. doi: 10.1007/BF01869986. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Stefani E., Chávez de Ramírez B. Occluding junctions of the Necturus gallbladder. J Membr Biol. 1982;70(1):15–25. doi: 10.1007/BF01871585. [DOI] [PubMed] [Google Scholar]
- Drouin H., The R. The effect of reducing extracellular pH on the membrane currents of the ranvier node. Pflugers Arch. 1969;313(1):80–88. doi: 10.1007/BF00586331. [DOI] [PubMed] [Google Scholar]
- Essig A. Influence of cellular and paracellular conductance patterns on epithelial transport and metabolism. Biophys J. 1982 May;38(2):143–152. doi: 10.1016/S0006-3495(82)84541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando Garcia-Diaz J., Corcia A., Armstrong W. M. Intracellular chloride activity and apical membrane chloride conductance in Necturus gallbladder. J Membr Biol. 1983;73(2):145–155. doi: 10.1007/BF01870438. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., Persson B. E., Spring K. R. Epithelial cell volume regulation: bicarbonate dependence. Science. 1981 Dec 18;214(4527):1357–1359. doi: 10.1126/science.7313695. [DOI] [PubMed] [Google Scholar]
- Garcia-Diaz J. F., Armstrong W. M. The steady-state relationship between sodium and chloride transmembrane electrochemical potential differences in Necturus gallbladder. J Membr Biol. 1980 Aug 7;55(3):213–222. doi: 10.1007/BF01869462. [DOI] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Schultz S. G. Potassium transport and intracellular potassium activities in rabbit gallbladder. J Membr Biol. 1982;65(1-2):41–47. doi: 10.1007/BF01870467. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Van Driessche W. Noise analysis of the K+ current through the apical membrane of Necturus gallbladder. J Membr Biol. 1981;60(3):187–198. doi: 10.1007/BF01992557. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Van Driessche W. The effect of electrical gradients on current fluctuations and impedance recorded from Necturus gallbladder. J Membr Biol. 1981;60(3):199–209. doi: 10.1007/BF01992558. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Blockade of voltage-dependent and Ca2+-dependent K+ current components by internal Ba2+ in molluscan pacemaker neurons. Experientia. 1979 Feb 15;35(2):229–231. doi: 10.1007/BF01920633. [DOI] [PubMed] [Google Scholar]
- Hermann A., Gorman A. L. Effects of tetraethylammonium on potassium currents in a molluscan neurons. J Gen Physiol. 1981 Jul;78(1):87–110. doi: 10.1085/jgp.78.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968 Feb;51(2):221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Vergara C., Hidalgo C. Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1982 Feb;79(3):805–809. doi: 10.1073/pnas.79.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W., Essig A. Relationship of transepithelial electrical potential to membrane potentials and conductance ratios in frog skin. J Membr Biol. 1982;69(2):125–136. doi: 10.1007/BF01872272. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Reuss L., Cheung L. Y., Grady T. P. Mechanisms of cation permeation across apical cell membrane of Necturus gallbladder: effects of luminal pH and divalent cations on K+ and Na+ permeability. J Membr Biol. 1981 Apr 30;59(3):211–224. doi: 10.1007/BF01875426. [DOI] [PubMed] [Google Scholar]
- Reuss L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder: III. Ionic permeability of the basolateral cell membrane. J Membr Biol. 1979 May 25;47(3):239–259. doi: 10.1007/BF01869080. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. I. Circuit analysis and steady-state effects of mucosal solution ionic substitutions. J Membr Biol. 1975 Dec 4;25(1-2):115–139. doi: 10.1007/BF01868571. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Electrical properties of the cellular transepithelial pathway in Necturus gallbladder. II. Ionic permeability of the apical cell membrane. J Membr Biol. 1975 Dec 4;25(1-2):141–161. doi: 10.1007/BF01868572. [DOI] [PubMed] [Google Scholar]
- Reuss L., Finn A. L. Mechanisms of voltage transients during current clamp in Necturus gallbladder. J Membr Biol. 1977 Dec 15;37(3-4):299–319. doi: 10.1007/BF01940937. [DOI] [PubMed] [Google Scholar]
- Reuss L., Weinman S. A., Grady T. P. Intracellular K+ activity and its relation to basolateral membrane ion transport in Necturus gallbladder epithelium. J Gen Physiol. 1980 Jul;76(1):33–52. doi: 10.1085/jgp.76.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Weinman S. A. Intracellular ionic activities and transmembrane electrochemical potential differences in gallbladder epithelium. J Membr Biol. 1979 Sep 14;49(4):345–362. doi: 10.1007/BF01868991. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R. A., Epel D., Carroll E. J., Jr, Yanagimachi R. Is calcium ionophore a universal activator for unfertilised eggs? Nature. 1974 Nov 1;252(5478):41–43. doi: 10.1038/252041a0. [DOI] [PubMed] [Google Scholar]
- Steinhardt R., Zucker R., Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977 Jul 1;58(1):185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Kottra G., Kampmann L., Frömter E. Square wave pulse analysis of cellular and paracellular conductance pathways in Necturus gallbladder epithelium. Pflugers Arch. 1982 Oct 1;394(4):302–312. doi: 10.1007/BF00583694. [DOI] [PubMed] [Google Scholar]
- Van Driessche W., Gögelein H. Potassium channels in the apical membrane of the toad gallbladder. Nature. 1978 Oct 19;275(5681):665–667. doi: 10.1038/275665a0. [DOI] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Wong B. S., Lecar H., Adler M. Single calcium-dependent potassium channels in clonal anterior pituitary cells. Biophys J. 1982 Sep;39(3):313–317. doi: 10.1016/S0006-3495(82)84522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Effects of pH and polyvalent cations on the selective permeability of gall-bladder epithelium to monovalent ions. Biochim Biophys Acta. 1968 Aug;163(1):57–74. doi: 10.1016/0005-2736(68)90033-3. [DOI] [PubMed] [Google Scholar]
- van Os C. H., Slegers J. F. The electrical potential profile of gallbladder epithelium. J Membr Biol. 1975 Dec 4;24(3-4):341–363. doi: 10.1007/BF01868631. [DOI] [PubMed] [Google Scholar]



