Abstract
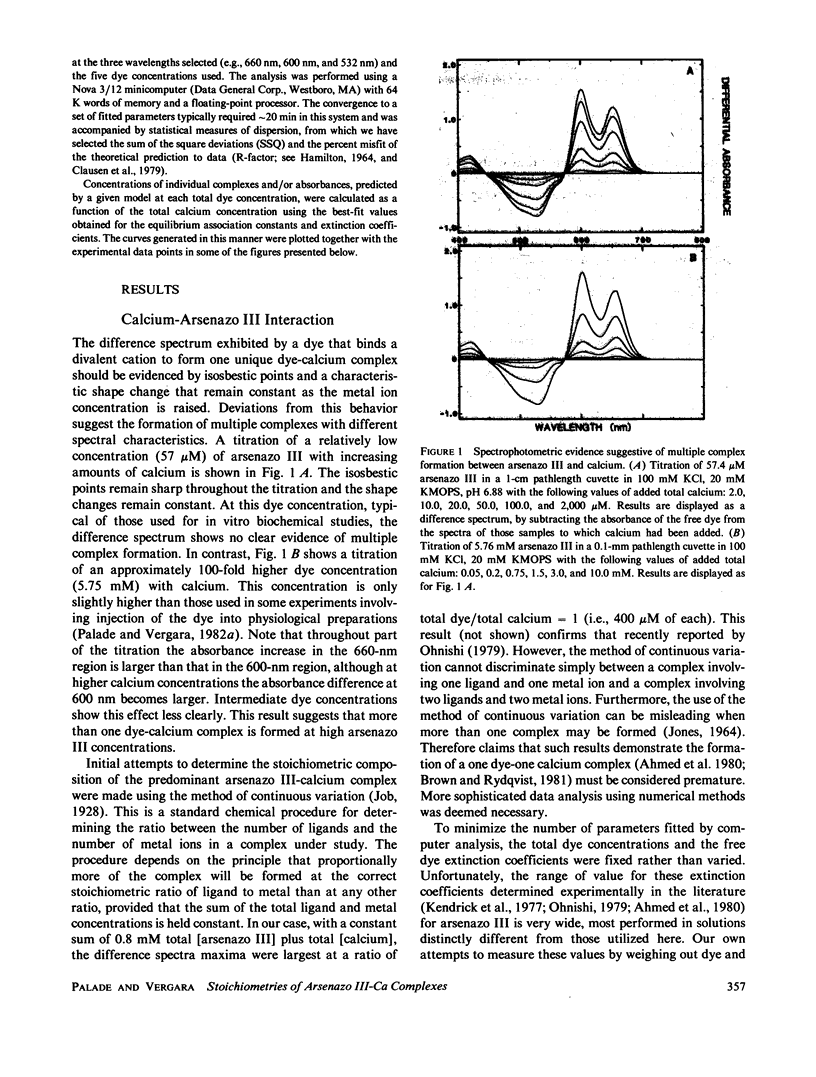
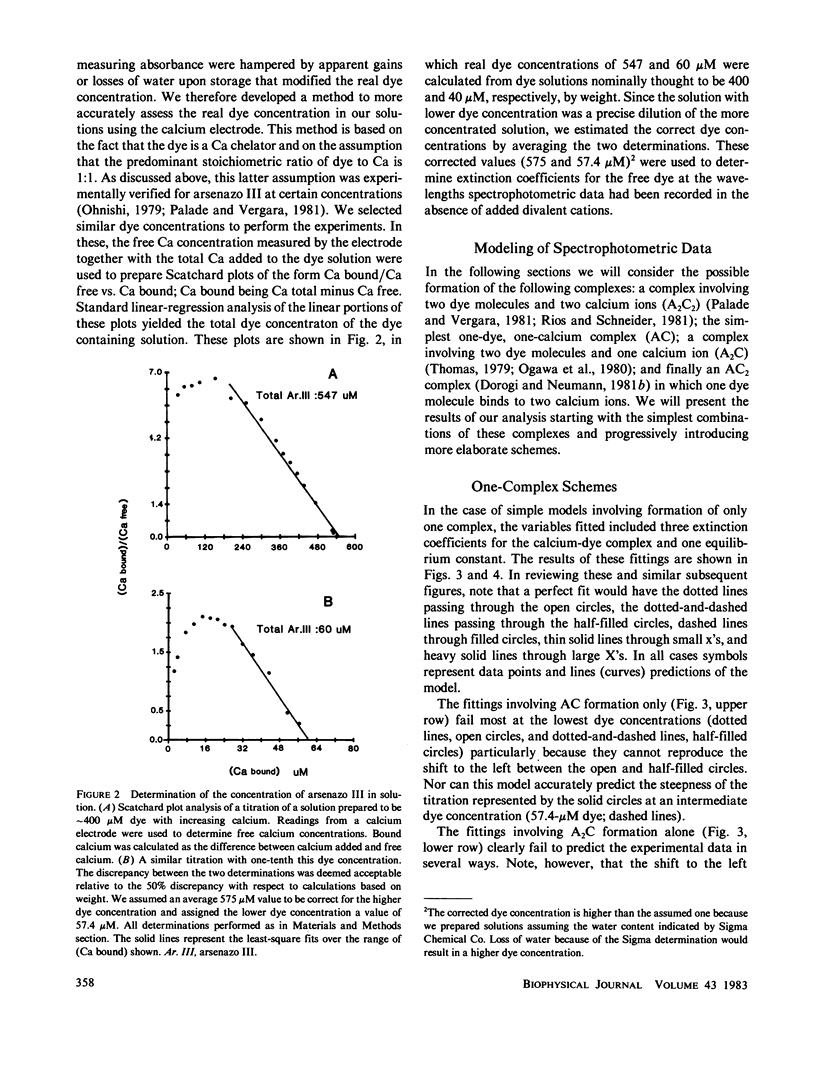
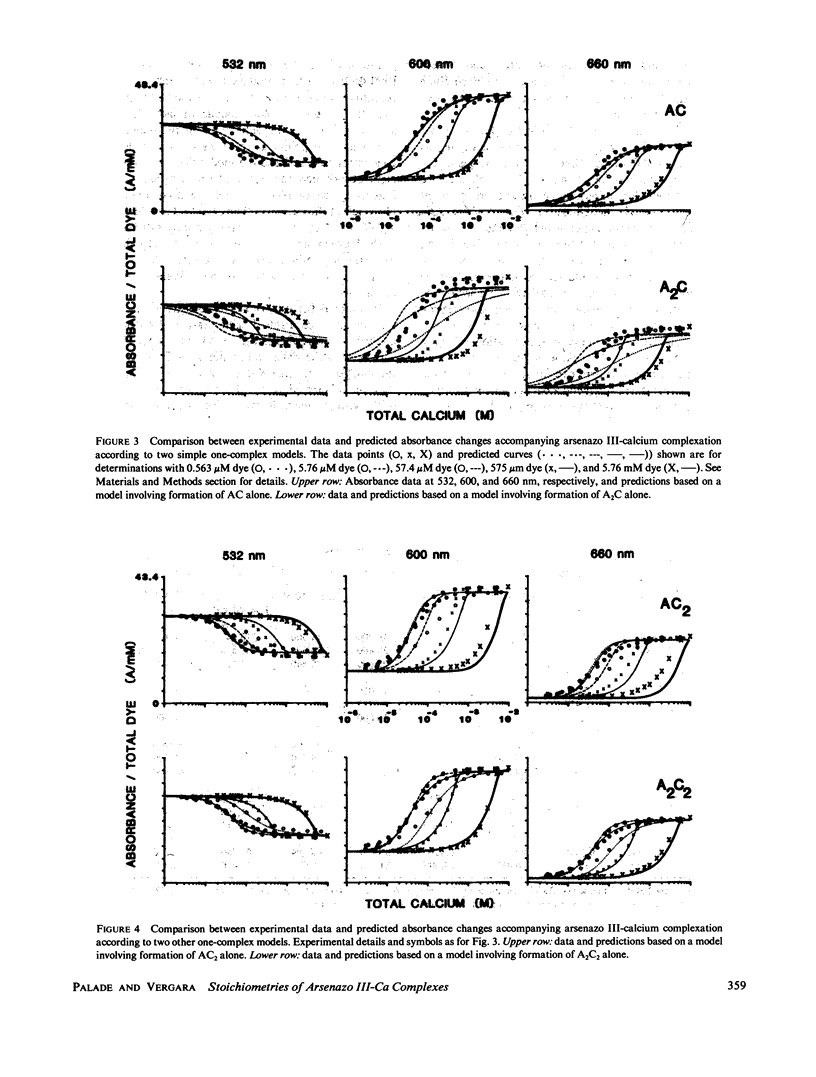
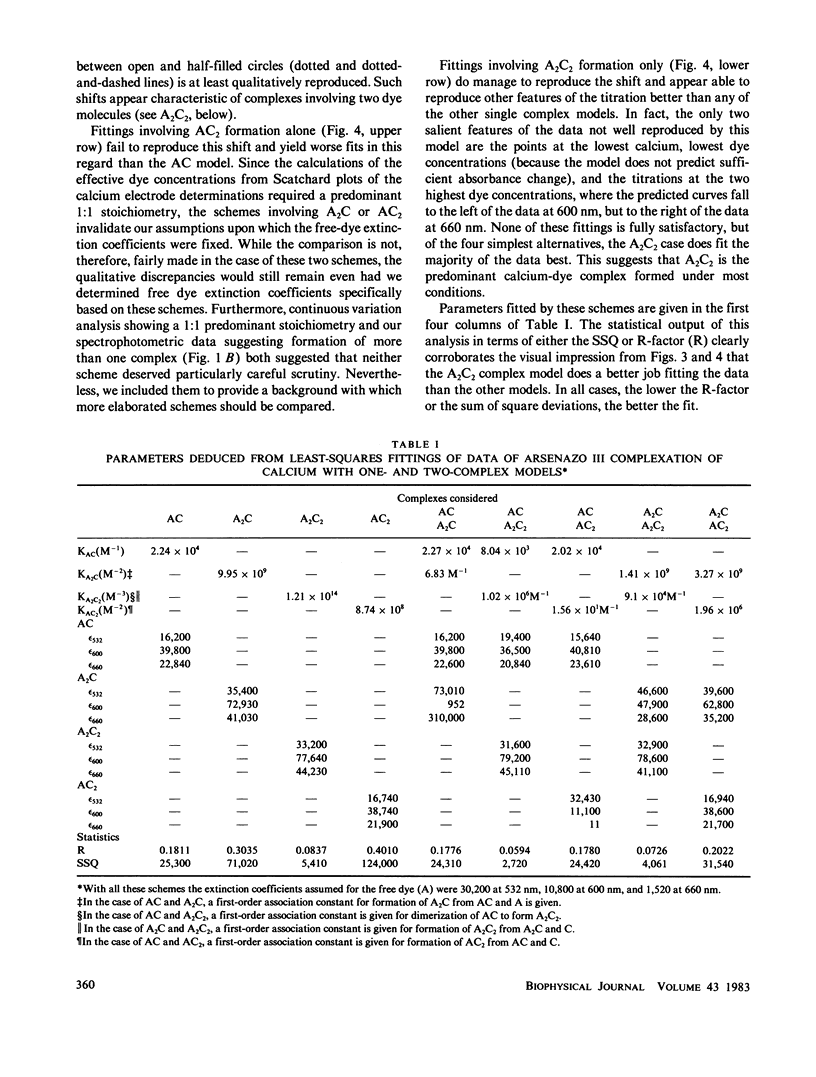
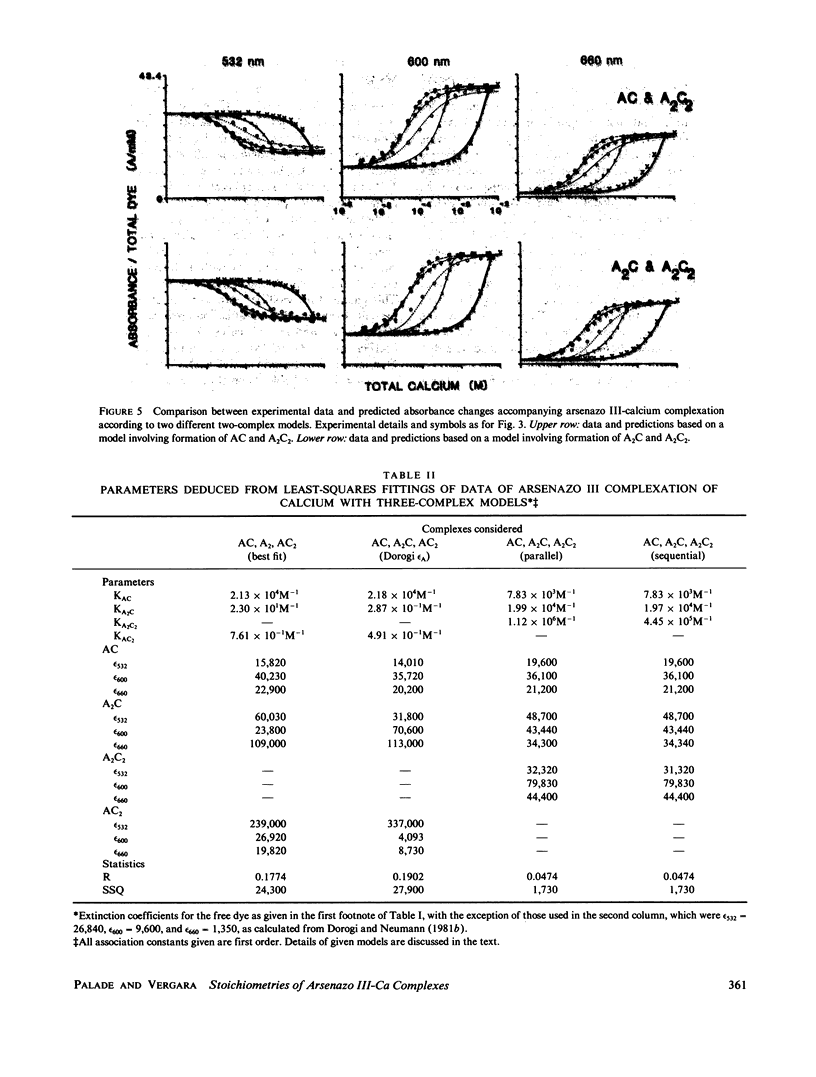
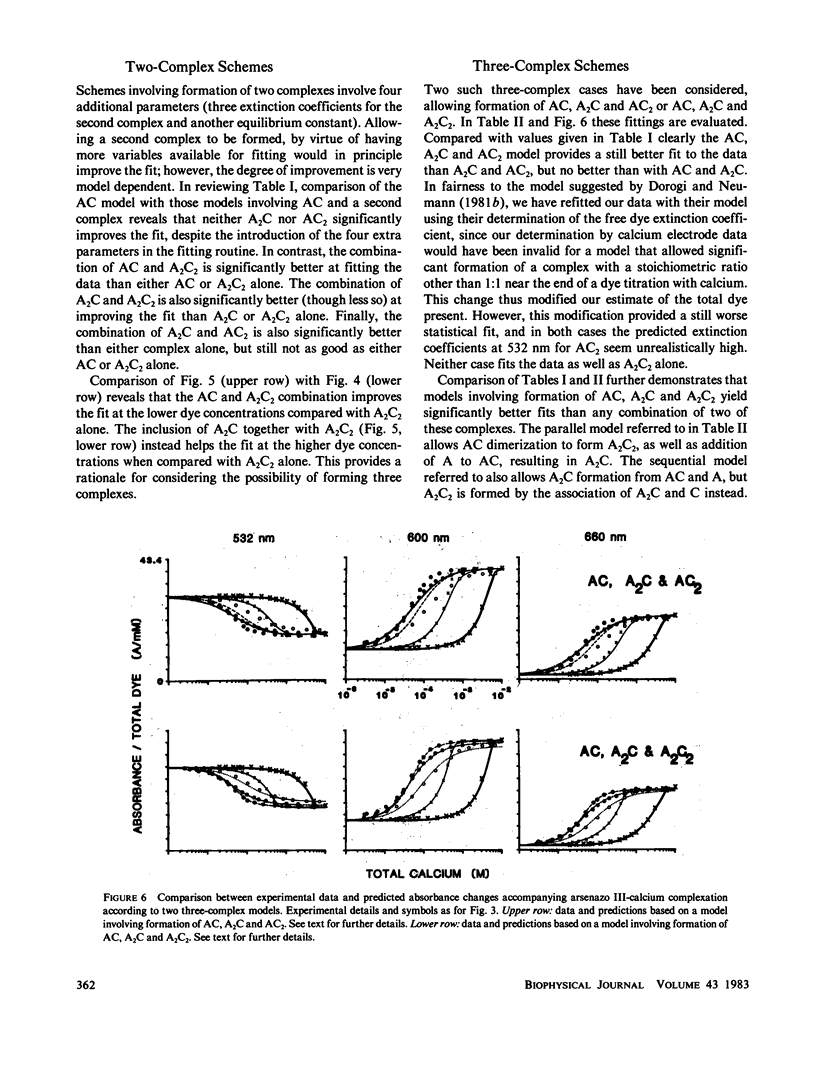
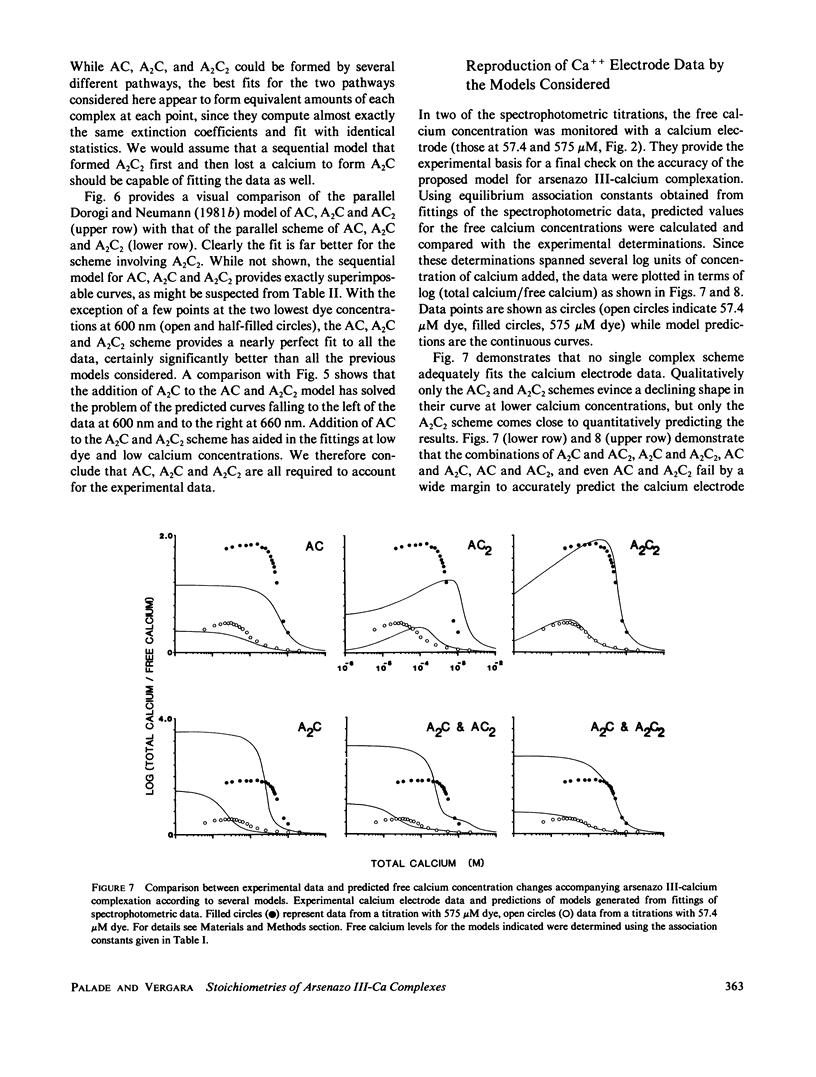
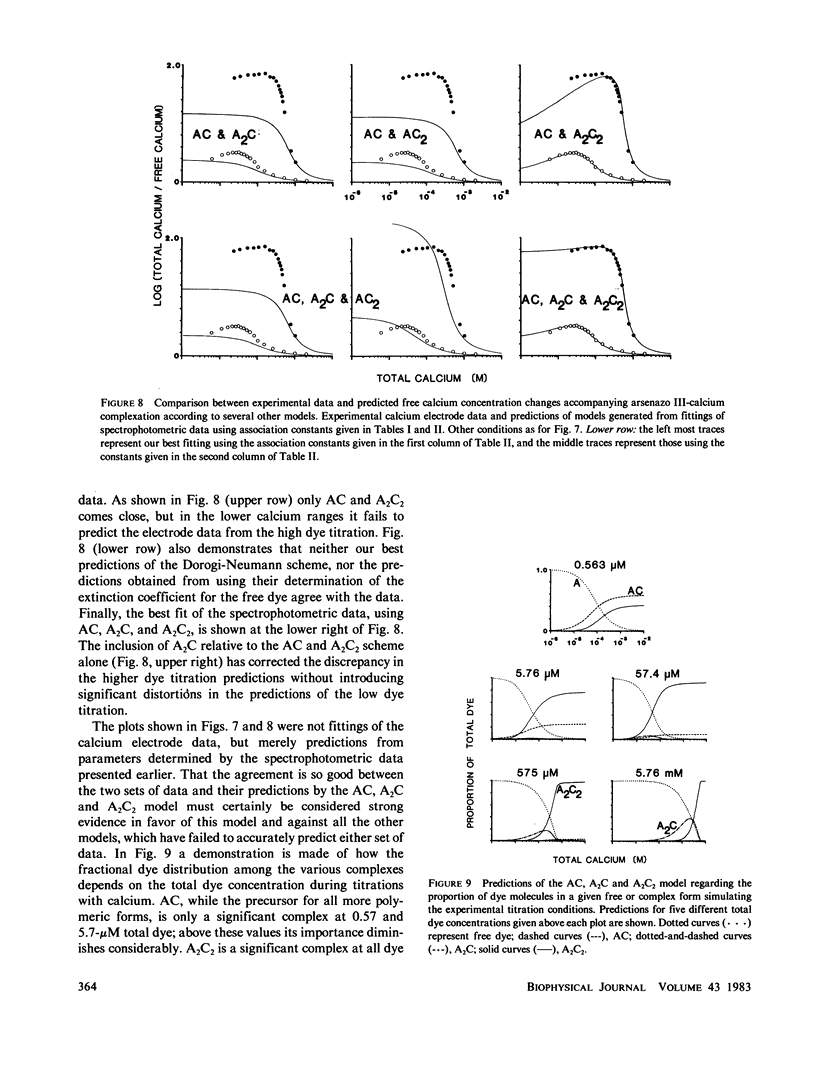
The equilibrium interactions of the metallochromic indicator arsenazo III with calcium at physiological ionic strength and pH were investigated spectrophotometrically and with the aid of a calcium electrode. Evidence suggests the formation of more than one dye-calcium complex. The analysis of data obtained over a 10,000-fold range of dye concentrations concludes that at the concentrations used for in vitro biochemical studies (10--100 microM), arsenazo III absorbance changes in response to calcium binding primarily involve the formation of a complex involving two dye molecules and two calcium ions. At millimolar dye concentrations, typical of physiological calcium transient determinations in situ, a second complex involving two arsenazo III molecules and one calcium ion is additionally formed. A third complex, involving one arsenazo III molecule and one calcium ion, is formed at very low dye concentrations. The results reported here suggest that equilibrium calibration of the dye with calcium cannot be used directly to satisfactorily relate transient absorbance changes in physiological preparations to calcium concentration changes since several stoichiometrically distinct complexes with different absorbances could be formed at different rates. The results of this study do not permit the elucidation of a unique kinetic scheme of arsenazo III complexation with calcium; for this, in vitro kinetic analysis is required. Results of similar analysis of the dye interaction with magnesium are also reported, and these appear compatible with a much simpler model of complexation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Kragie L., Connor J. A. Stoichiometry and apparent dissociation constant of the calcium-arsenazo III reaction under physiological conditions. Biophys J. 1980 Dec;32(3):907–920. doi: 10.1016/S0006-3495(80)85025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P. J. Affinity and stoichiometry of calcium binding by arsenazo III. Anal Biochem. 1981 Jan 1;110(1):61–72. doi: 10.1016/0003-2697(81)90112-3. [DOI] [PubMed] [Google Scholar]
- Baylor S. M., Chandler W. K., Marshall M. W. Use of metallochromic dyes to measure changes in myoplasmic calcium during activity in frog skeletal muscle fibres. J Physiol. 1982 Oct;331:139–177. doi: 10.1113/jphysiol.1982.sp014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler T. J., Schibeci A., Martonosi A. The binding of arsenazo III to cell components. Biochim Biophys Acta. 1980 May 7;629(2):317–327. doi: 10.1016/0304-4165(80)90104-x. [DOI] [PubMed] [Google Scholar]
- Brown H. M., Rydqvist B. Arsenazo III-Ca2+. Effect of pH, ionic strength, and arsenazo III concentration on equilibrium binding evaluated with Ca2+ ion-sensitive electrodes and absorbance measurements. Biophys J. 1981 Oct;36(1):117–137. doi: 10.1016/S0006-3495(81)84720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu V. C., Haynes D. The pH dependence and the binding equilibria of the calcium indicator--arsenazo III. Membr Biochem. 1980;3(3):169–183. doi: 10.3109/09687688009063884. [DOI] [PubMed] [Google Scholar]
- Clausen C., Lewis S. A., Diamond J. M. Impedance analysis of a tight epithelium using a distributed resistance model. Biophys J. 1979 May;26(2):291–317. doi: 10.1016/S0006-3495(79)85250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorogi P. L., Neumann E. Spectrophotometric determination of reaction stoichiometry and equilibrium constants of metallochromic indicators. I. General calculational method. Biophys Chem. 1981 Apr;13(2):117–123. doi: 10.1016/0301-4622(81)80010-5. [DOI] [PubMed] [Google Scholar]
- Dorogi P. L., Neumann E. Spectrophotometric determination of reaction stoichiometry and equilibrium constants of metallochromic indicators. II. The Ca2+-arsenazo III complexes. Biophys Chem. 1981 Apr;13(2):125–131. doi: 10.1016/0301-4622(81)80011-7. [DOI] [PubMed] [Google Scholar]
- Kendrick N. C., Ratzlaff R. W., Blaustein M. P. Arsenazo III as an indicator for ionized calcium in physiological salt solutions: its use for determination of the CaATP dissociation constant. Anal Biochem. 1977 Dec;83(2):433–450. doi: 10.1016/0003-2697(77)90052-5. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Calcium transients in frog slow muscle fibres. Nature. 1977 Aug 25;268(5622):750–752. doi: 10.1038/268750a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Calcium transients in normal and denervated slow muscle fibres of the frog. J Physiol. 1981 Sep;318:191–206. doi: 10.1113/jphysiol.1981.sp013858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of calcium transients in frog muscle by the use of arsenazo III. Proc R Soc Lond B Biol Sci. 1977 Aug 22;198(1131):201–210. doi: 10.1098/rspb.1977.0094. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transition temperature of excitation-contraction coupling in frog twitch muscle fibres. Nature. 1979 Jul 26;280(5720):326–328. doi: 10.1038/280326a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Transmitter induced calcium entry across the post-synaptic membrane at frog end-plates measured using arsenazo III. J Physiol. 1980 Mar;300:197–212. doi: 10.1113/jphysiol.1980.sp013158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogan K., Simons E. R. The influence of pH on arsenazo III. Anal Biochem. 1979 Jul 1;96(1):70–76. doi: 10.1016/0003-2697(79)90555-4. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Harafuji H., Kurebayashi N. Comparison of the characteristics of four metallochromic dyes as potential calcium indicators for biological experiments. J Biochem. 1980 May;87(5):1293–1303. doi: 10.1093/oxfordjournals.jbchem.a132867. [DOI] [PubMed] [Google Scholar]
- Ohnishi S. T. A method of estimating the amount of calcium bound to the metallochromic indicator arsenazo III. Biochim Biophys Acta. 1979 Aug 22;586(2):217–230. doi: 10.1016/0304-4165(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Palade P., Vergara J. Arsenazo III and antipyrylazo III calcium transients in single skeletal muscle fibers. J Gen Physiol. 1982 Apr;79(4):679–707. doi: 10.1085/jgp.79.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Schneider M. F. Stoichiometry of the reactions of calcium with the metallochromic indicator dyes antipyrylazo III and arsenazo III. Biophys J. 1981 Dec;36(3):607–621. doi: 10.1016/S0006-3495(81)84755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A. Measurements of cation transport with metallochromic indicators. Methods Enzymol. 1979;56:301–338. doi: 10.1016/0076-6879(79)56030-3. [DOI] [PubMed] [Google Scholar]
- Thomas M. V. Arsenazo III forms 2:1 complexes with Ca and 1:1 complexes with Mg under physiological conditions. Estimates of the apparent dissociation constants. Biophys J. 1979 Mar;25(3):541–548. doi: 10.1016/S0006-3495(79)85322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


