Abstract
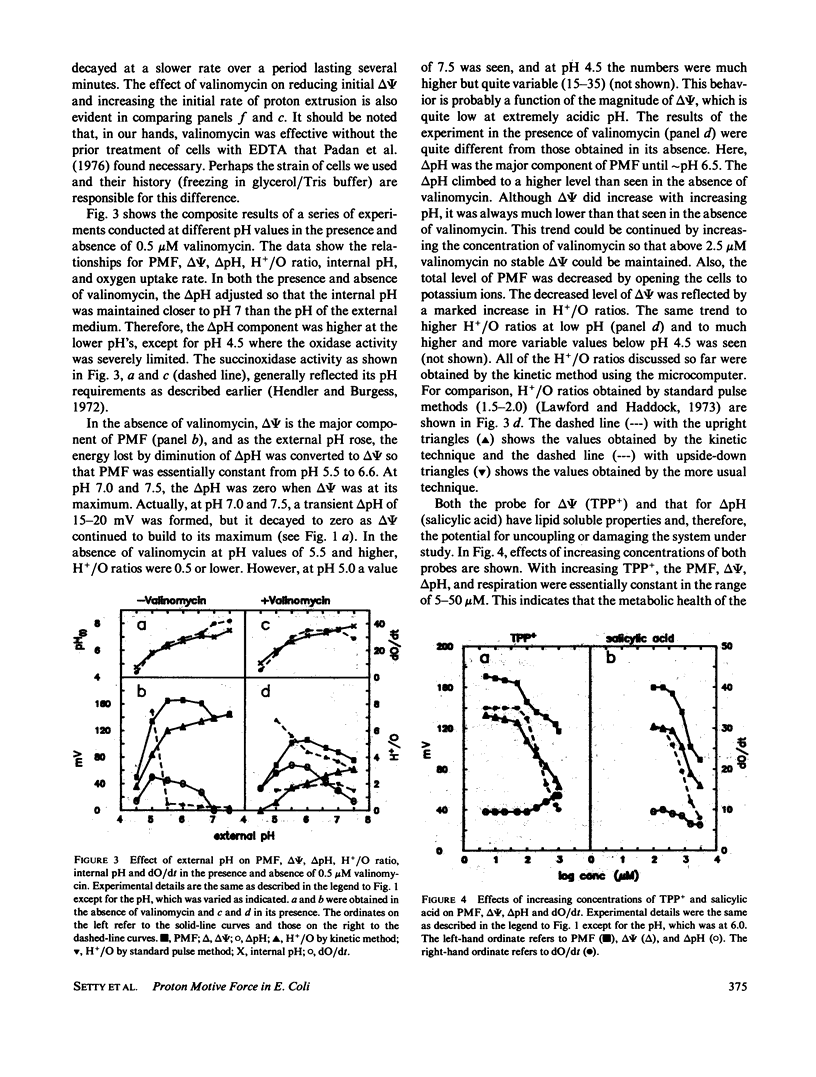
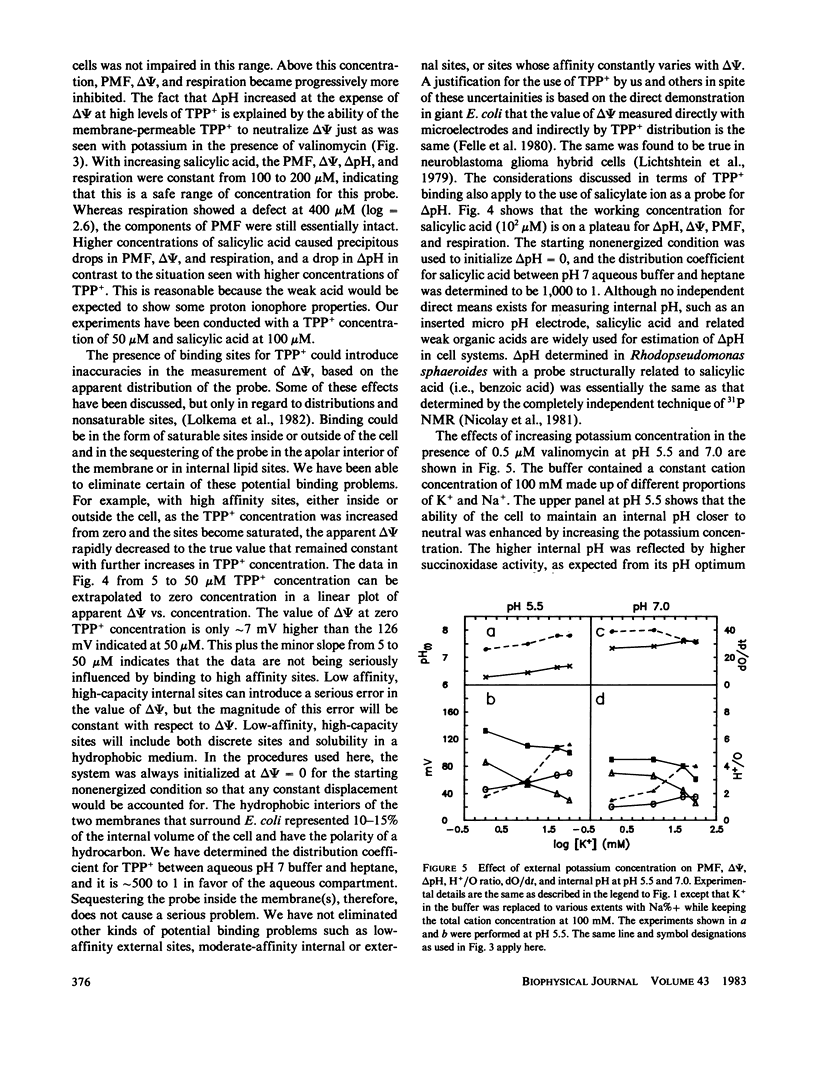
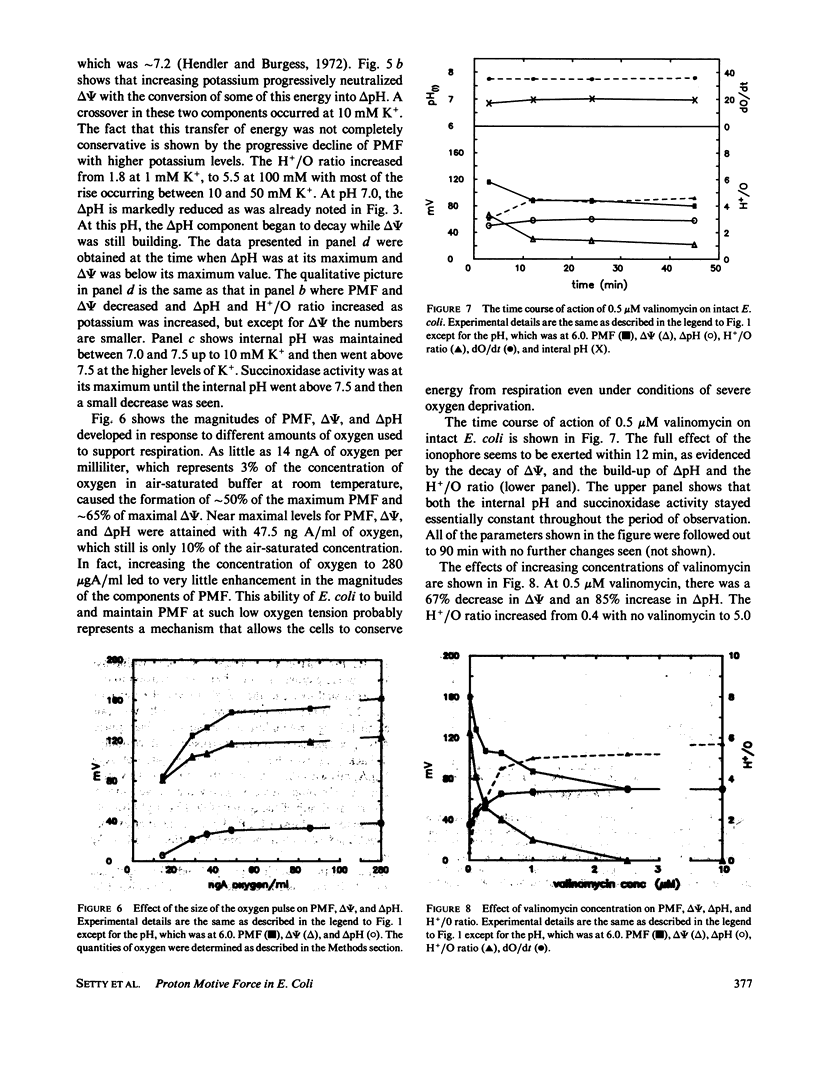
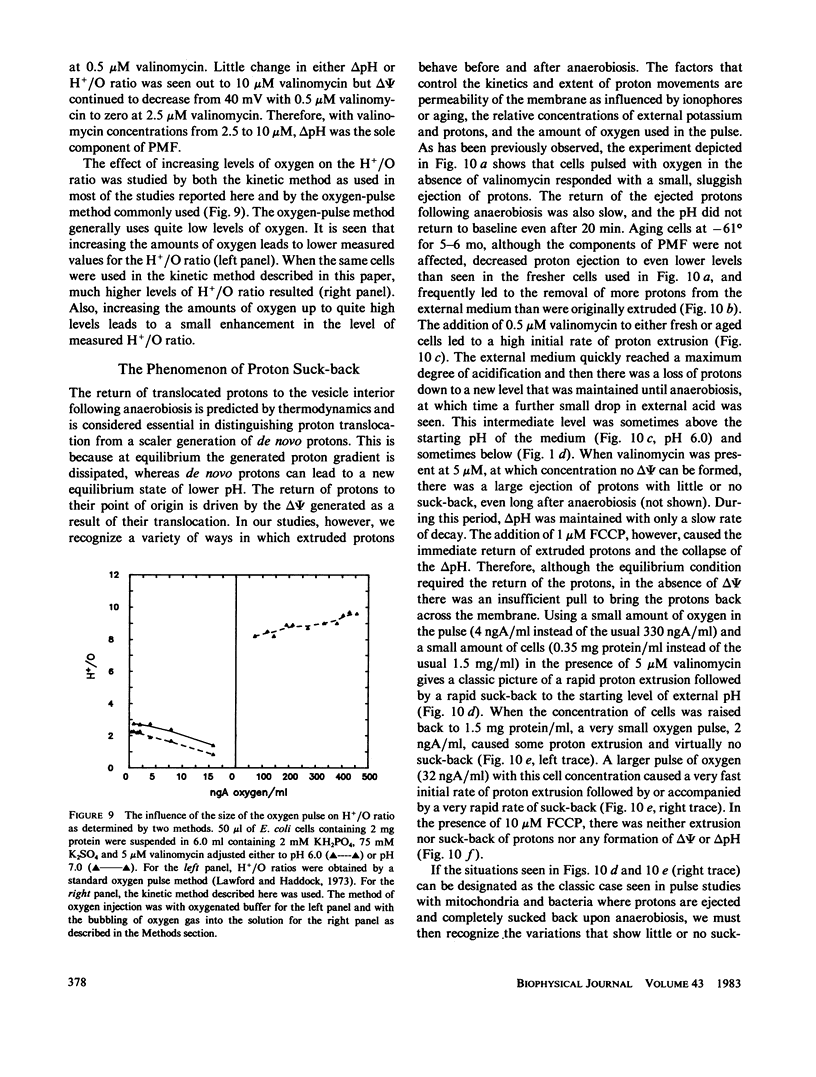
An instrument is described that enables the simultaneous monitoring of proton motive force (PMF), membrane potential (delta psi), the delta pH across a membrane, oxidase activity, proton movements, and H+/O ratios. We have studied the relationship existing among these parameters of energy transduction as a critical condition is changed during an experiment. The major findings are: (a) In the pH range of 4.5 to 7.5, increasing the external pH causes an increase in delta psi, internal pH, and oxidase activity, a decrease in H+/O ratio, and a peak-plateau in PMF from pH 5.5 to 6.6 where delta pH is converted to delta psi. (b) An increase in [K+] from 1 to 100 mM, in the presence of 0.5 microM valinomycin, causes the conversion of delta psi to delta pH, a gradual decline in PMF and an increase in H+/O ratio, internal pH, and oxidase activity. (c) Increasing valinomycin concentration from 0 to 2.5 microM, in the presence of 50 mM [K+], causes a decline in delta psi from 125 to 0 mV, and an increase in delta pH from 35 to 70 mV. From 2.5 to 10 microM, the delta pH and the PMF (which it solely represents), stay constant, H+/O ratio increases mainly from 0 to 0.5 microM and much more slowly from 2.5 to 10 microM. (d) Oxygen at only 10% of its concentration in air-saturated buffer can support the generation of 90% or more of the delta psi, delta pH, and PMF generated in an air-saturated solution. (e) The return of extruded protons to the cell (referred to here as "suck-back") represents a complicated process driven by delta psi and influenced by a variety of factors. (f) H+/O ratios measured by the kinetic technique used here are much higher than those measured by standard oxygen pulse techniques.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth I. R., Mitchell W. J., Hamilton W. A. Quantitative analysis of proton-linked transport systems. The lactose permease of Escherichia coli. Biochem J. 1979 Sep 15;182(3):687–696. doi: 10.1042/bj1820687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Stoichiometric relationship between energy-dependent proton ejection and electron transport in mitochondria. Proc Natl Acad Sci U S A. 1976 Feb;73(2):437–441. doi: 10.1073/pnas.73.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookman J. J., Downie J. A., Gibson F., Cox G. B., Rosenberg H. Proton translocation in cytochrome-deficient mutants of Escherichia coli. J Bacteriol. 1979 Feb;137(2):705–710. doi: 10.1128/jb.137.2.705-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Respiration and protein synthesis in Escherichia coli membrane-envelope fragments. VI. Solubilization and characterization of the electron transport chain. J Cell Biol. 1972 Nov;55(2):266–281. doi: 10.1083/jcb.55.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Kroll R. G., Booth I. R. The role of potassium transport in the generation of a pH gradient in Escherichia coli. Biochem J. 1981 Sep 15;198(3):691–698. doi: 10.1042/bj1980691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawford H. G., Haddock B. A. Respiration-driven proton translocation in Escherichia coli. Biochem J. 1973 Sep;136(1):217–220. doi: 10.1042/bj1360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiration-driven proton translocation in rat liver mitochondria. Biochem J. 1967 Dec;105(3):1147–1162. doi: 10.1042/bj1051147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. The Ninth Sir Hans Krebs Lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur J Biochem. 1979 Mar 15;95(1):1–20. doi: 10.1111/j.1432-1033.1979.tb12934.x. [DOI] [PubMed] [Google Scholar]
- Nicolay K., Lolkema J., Hellingwerf K. J., Kaptein R., Konings W. N. Quantitative agreement between the values for the light-induced delta pH in Rhodopseudomonas sphaeroides measured with automated follow-dialysis and 31P NMR. FEBS Lett. 1981 Jan 26;123(2):319–323. doi: 10.1016/0014-5793(81)80318-3. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. pH-dependent changes in proton:substrate stoichiometries during active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Sep 20;16(19):4270–4275. doi: 10.1021/bi00638a022. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynafarje B., Alexandre A., Davies P., Lehninger A. L. Proton translocation stoichiometry of cytochrome oxidase: use of a fast-responding oxygen electrode. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7218–7222. doi: 10.1073/pnas.79.23.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]
- Wikström M., Krab K. Proton-pumping cytochrome c oxidase. Biochim Biophys Acta. 1979 Aug 17;549(2):177–122. doi: 10.1016/0304-4173(79)90014-4. [DOI] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]


