Abstract
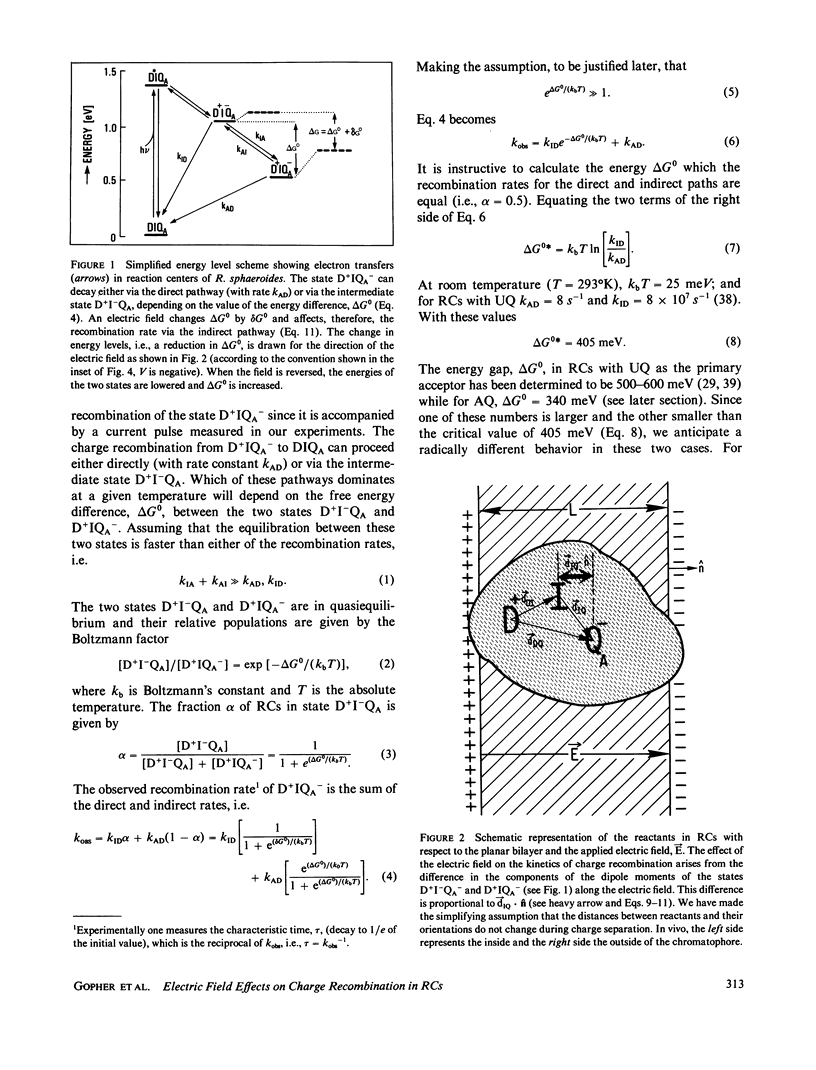
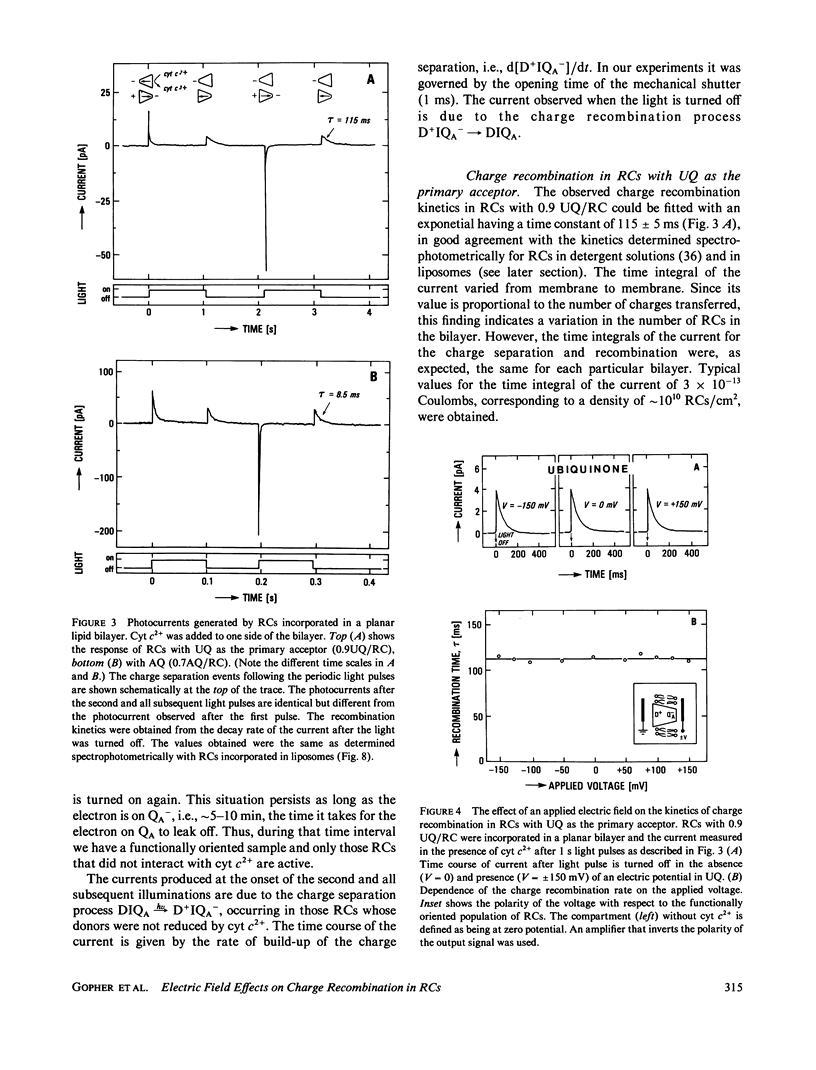
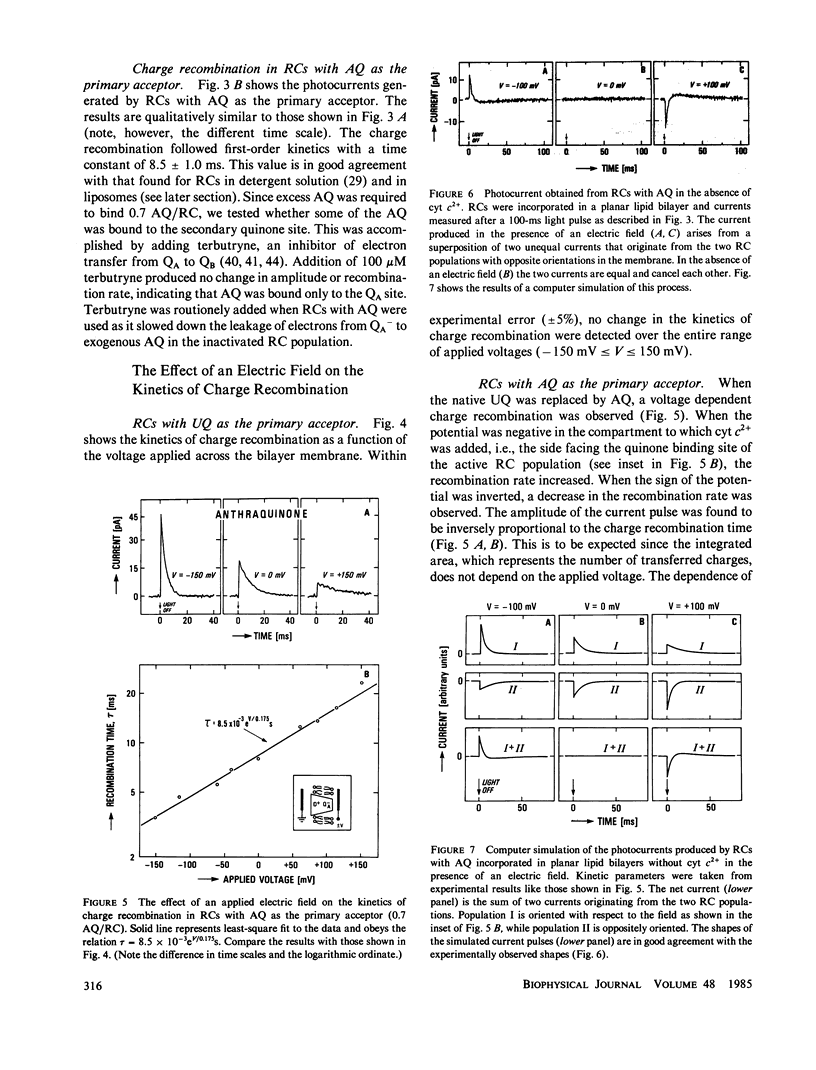
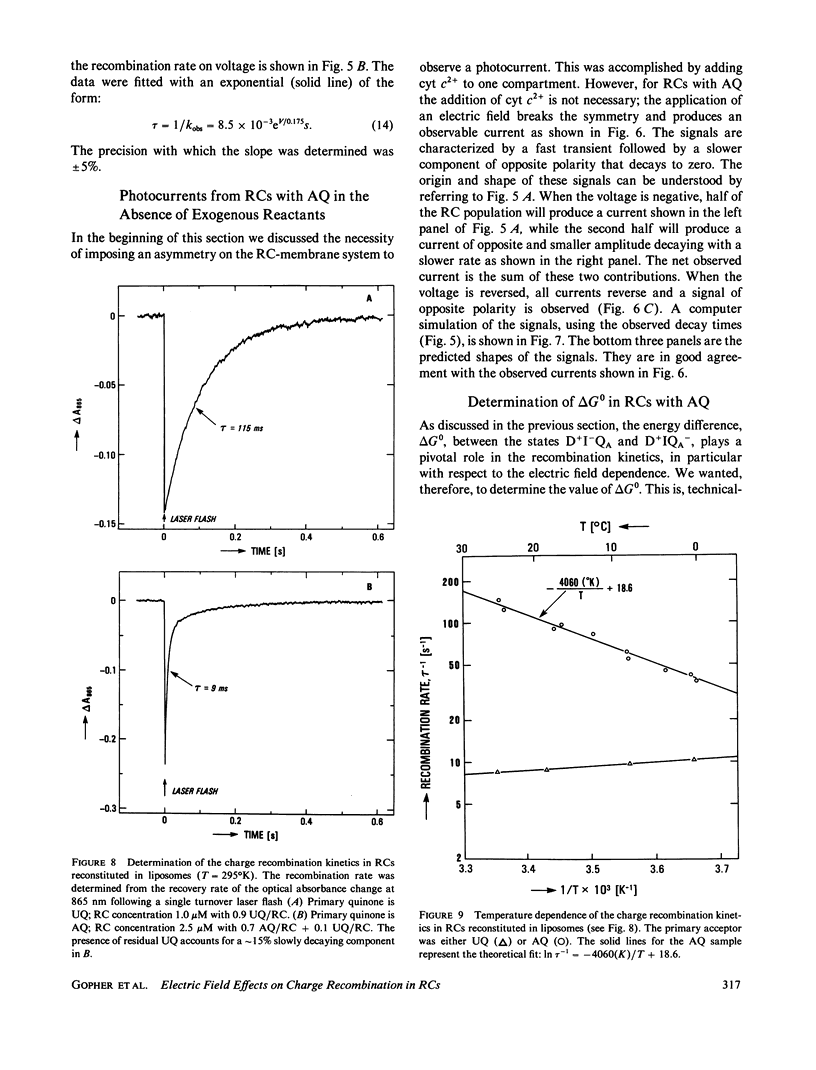
Reaction Centers (RCs) from the photosynthetic bacterium Rhodopseudomonas sphaeroides were incorporated in planar bilayers made from monolayers derived from liposomes reconstituted with purified RCs. The photocurrents associated with the charge recombination process between the reduced primary quinone (QA-) and the oxidized bacteriochlorophyll donor (D+) were measured as a function of voltage (-150 mV less than V less than 150 mV) applied across the bilayer. When QA was the native ubiquinone (UQ) the charge recombination was voltage independent. However, when UQ was replaced by anthraquinone (AQ), the recombination time depended on the applied voltage V according to the relation tau = 8.5 X 10(-3) eV/0.175S. These results were explained by a simple model in which the charge recombination from UQ- proceeds directly to D+ while that from AQ occurs via a thermally activated intermediate state, D+I-QA, where I is the intermediate acceptor. The voltage dependence arises from an electric field induced change in the energy gap, delta G0, between the states D+I-QA and D+IQA-. This model is supported by the measured temperature dependence of the charge recombination time, which for RCs with AQ gave a value of delta G0 = 340 +/- 20 meV. In contrast, delta G0 for RCs with UQ as the primary acceptor, is sufficiently large (approximately 550 meV) so that even in the presence of the field, the direct pathway dominates. The voltage dependence shows that the electron transfer from I- to QA is electrogenic. From a quantitative analysis of the voltage dependence on the recombination rate it was concluded that the component of the distance between I and QA along the normal to the membrane is about one-seventh of the thickness of the membrane. This implies that the electron transfer from I to Q contributes at least one-seventh to the potential generated by the charge separation between D+ and QA-.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsky E. L., Dancshazy Z., Drachey L. A., Il'ina M. D., Jasaitis A. A., Kondrashin A. A., Samuilov V. D., Skulachev V. P. Reconstitution of biological molecular generators of electric current. Bacteriochlorophyll and plant chlorophyll complexes. J Biol Chem. 1976 Nov 25;251(22):7066–7071. [PubMed] [Google Scholar]
- Crofts A. R., Wraight C. A., Fleischmann D. E. Energy conservation in the photochemical reactions of photosynthesis and its relation to delayed fluorescence. FEBS Lett. 1971 Jun 10;15(2):89–100. doi: 10.1016/0014-5793(71)80031-5. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Semenov AYu, Skulachev V. P., Smirnova I. A., Chamorovsky S. K., Kononenko A. A., Rubin A. B., Uspenskaya NYa Fast stages of photoelectric processes in biological membranes. III. Bacterial photosynthetic redox system. Eur J Biochem. 1981 Jul;117(3):483–489. doi: 10.1111/j.1432-1033.1981.tb06363.x. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Crofts A. R. The high energy state in chromatophores from Rhodopseudomonas spheroides. FEBS Lett. 1969 Aug;4(3):185–189. doi: 10.1016/0014-5793(69)80230-9. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Dutton P. L. The kinetic and redox potentiometric resolution of the carotenoid shifts in Rhodopseudomonas spheroides chromatophores: their relationship to electric field alterations in electron transport and energy coupling. Biochim Biophys Acta. 1973 Oct 19;325(1):102–113. doi: 10.1016/0005-2728(73)90155-2. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D., Okamura M. Y., Feher G. Electron transfer in reaction centers of Rhodopseudomonas sphaeroides. I. Determination of the charge recombination pathway of D+QAQ(-)B and free energy and kinetic relations between Q(-)AQB and QAQ(-)B. Biochim Biophys Acta. 1984 Jul 27;766(1):126–140. doi: 10.1016/0005-2728(84)90224-x. [DOI] [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Spectroscopic and kinetic properties of the transient intermediate acceptor in reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1979 Jun 5;546(3):394–417. doi: 10.1016/0005-2728(79)90076-8. [DOI] [PubMed] [Google Scholar]
- Pachence J. M., Dutton P. L., Blasie J. K. Structural studies on reconstituted reaction center-phosphatidylcholine membranes. Biochim Biophys Acta. 1979 Nov 8;548(2):348–373. doi: 10.1016/0005-2728(79)90141-5. [DOI] [PubMed] [Google Scholar]
- Packham N. K., Dutton P. L., Mueller P. Photoelectric currents across planar bilayer membranes containing bacterial reaction centers. Response under conditions of single electron turnover. Biophys J. 1982 Feb;37(2):465–473. doi: 10.1016/S0006-3495(82)84693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham N. K., Packham C., Mueller P., Tiede D. M., Dutton P. L. Reconstitution of photochemically active reaction centers in planar phospholipid membranes. Light-induced electrical currents under voltage-clamped conditions. FEBS Lett. 1980 Jan 28;110(1):101–106. doi: 10.1016/0014-5793(80)80033-0. [DOI] [PubMed] [Google Scholar]
- Peters K., Avouris P., Rentzepis P. M. Picosecond dynamics of primary electron-transfer processes in bacterial photosynthesis. Biophys J. 1978 Aug;23(2):207–217. doi: 10.1016/S0006-3495(78)85443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- Schindler H. Formation of planar bilayers from artificial or native membrane vesicles. FEBS Lett. 1980 Dec 15;122(1):77–79. doi: 10.1016/0014-5793(80)80405-4. [DOI] [PubMed] [Google Scholar]
- Schönfeld M., Montal M., Feher G. Functional reconstitution of photosynthetic reaction centers in planar lipid bilayers. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6351–6355. doi: 10.1073/pnas.76.12.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfeld M., Montal M., Feher G. Reaction center--phospholipid complex in organic solvents: formation and properties. Biochemistry. 1980 Apr 15;19(8):1535–1542. doi: 10.1021/bi00549a001. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Parson W. W. Energies and kinetics of radical pairs involving bacteriochlorophyll and bacteriopheophytin in bacterial reaction centers. Proc Natl Acad Sci U S A. 1981 Feb;78(2):957–961. doi: 10.1073/pnas.78.2.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein R. R., Castellvi A. L., Bogacz J. P., Wraight C. A. Herbicide-quinone competition in the acceptor complex of photosynthetic reaction centers from Rhodopseudomonas sphaeroides: a bacterial model for PS-II-herbicide activity in plants. J Cell Biochem. 1984;24(3):243–259. doi: 10.1002/jcb.240240306. [DOI] [PubMed] [Google Scholar]
- Trissl H. W. Spatial correlation between primary redox components in reaction centers of Rhodopseudomonas sphaeroides measured by two electrical methods in the nanosecond range. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7173–7177. doi: 10.1073/pnas.80.23.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkirs G. E., Feher G. Topography of reaction center subunits in the membrane of the photosynthetic bacterium, rhodopseudomonas sphaeroides. J Cell Biol. 1982 Oct;95(1):179–188. doi: 10.1083/jcb.95.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G., Simon M. I. Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7303–7307. doi: 10.1073/pnas.81.23.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]


