Abstract
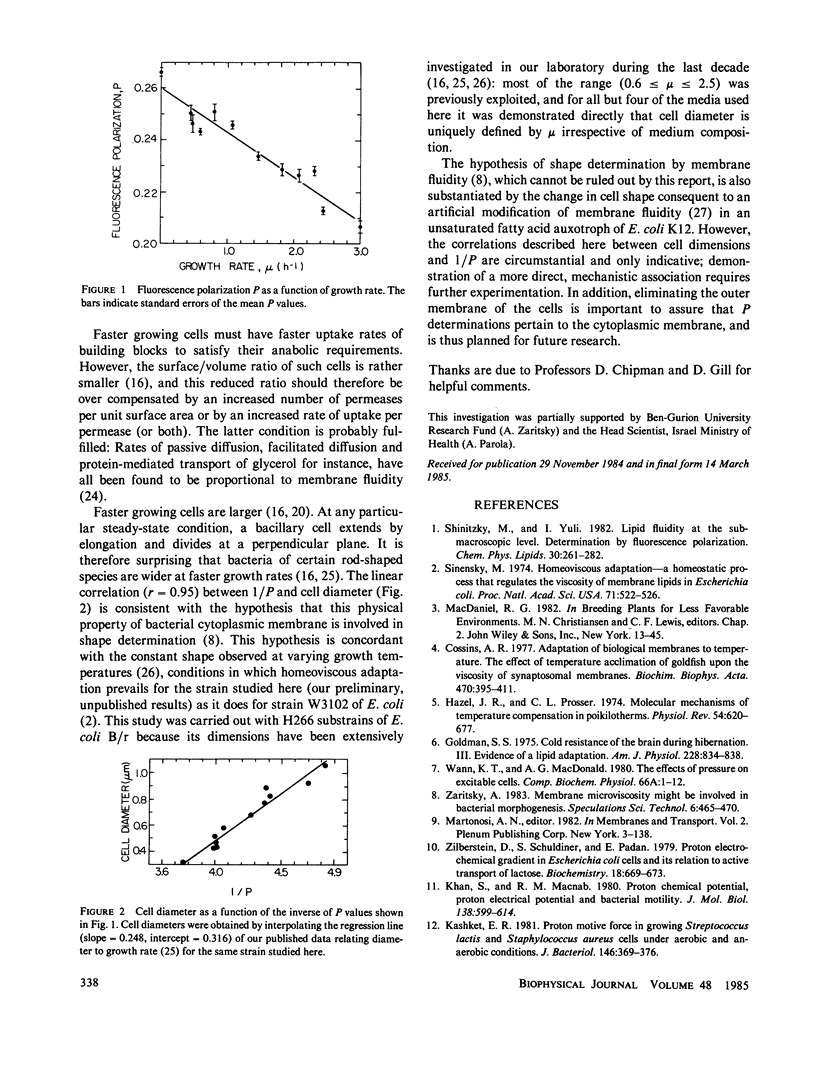
Fluorescence polarization, P, of 1,6-diphenyl-1,3,5-hexatriene was studied in Escherichia coli B/r. Modification of nutritional conditions was not compensated by homeoviscous adaptation, demonstrated to exist for temperature variations. Cell diameter, which is known also to vary with nutrition but not with temperature, was found to be positively correlated with 1/P, and may therefore be regulated by membrane lipid order and fluidity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cossins A. R. Adaptation of biological membranes to temperature. The effect of temperature acclimation of goldfish upon the viscosity of synaptosomal membranes. Biochim Biophys Acta. 1977 Nov 1;470(3):395–411. doi: 10.1016/0005-2736(77)90131-6. [DOI] [PubMed] [Google Scholar]
- Eze M. O., McElhaney R. N. The effect of alterations in the fluidity and phase state of the membrane lipids on the passive permeation and facilitated diffusion of glycerol in Escherichia coli. J Gen Microbiol. 1981 Jun;124(2):299–307. doi: 10.1099/00221287-124-2-299. [DOI] [PubMed] [Google Scholar]
- Goldman S. S. Cold resistance of the brain during hibernation. III. Evidence of a lipid adaptation. Am J Physiol. 1975 Mar;228(3):834–838. doi: 10.1152/ajplegacy.1975.228.3.834. [DOI] [PubMed] [Google Scholar]
- Hazel J. R., Prosser C. L. Molecular mechanisms of temperature compensation in poikilotherms. Physiol Rev. 1974 Jul;54(3):620–677. doi: 10.1152/physrev.1974.54.3.620. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Effects of aerobiosis and nitrogen source on the proton motive force in growing Escherichia coli and Klebsiella pneumoniae cells. J Bacteriol. 1981 Apr;146(1):377–384. doi: 10.1128/jb.146.1.377-384.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. Proton motive force in growing Streptococcus lactis and Staphylococcus aureus cells under aerobic and anaerobic conditions. J Bacteriol. 1981 Apr;146(1):369–376. doi: 10.1128/jb.146.1.369-376.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. Proton chemical potential, proton electrical potential and bacterial motility. J Mol Biol. 1980 Apr 15;138(3):599–614. doi: 10.1016/s0022-2836(80)80019-2. [DOI] [PubMed] [Google Scholar]
- Kuriki Y. Effects of unsaturated fatty acids on the morphogenesis of an unsaturated fatty acid auxotroph of Escherichia coli. J Bacteriol. 1981 Sep;147(3):1121–1124. doi: 10.1128/jb.147.3.1121-1124.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan I., Fleischer G., Livne A., Dvilansky A., Parola A. H. Membrane microenvironmental changes during activation of human blood platelets by thrombin. A study with a fluorescent probe. J Biol Chem. 1979 Oct 10;254(19):9822–9828. [PubMed] [Google Scholar]
- Newman E. B., Batist G., Fraser J., Isenberg S., Weyman P., Kapoor V. The use of glycine as nitrogen source by Escherichia coli K12. Biochim Biophys Acta. 1976 Jan 14;421(1):97–105. doi: 10.1016/0304-4165(76)90173-2. [DOI] [PubMed] [Google Scholar]
- Rosenberger R. F., Grover N. B., Zaritsky A., Woldringh C. L. Control of microbial surface-growth by density. Nature. 1978 Jan 19;271(5642):244–245. doi: 10.1038/271244a0. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Homeoviscous adaptation--a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Feb;71(2):522–525. doi: 10.1073/pnas.71.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueba F. J., van Spronsen E. A., Traas J., Woldringh C. L. Effects of temperature on the size and shape of Escherichia coli cells. Arch Microbiol. 1982 May;131(3):235–240. doi: 10.1007/BF00405885. [DOI] [PubMed] [Google Scholar]
- Zaritsky A. Effects of growth temperature on ribosomes and other physiological properties of Escherichia coli. J Bacteriol. 1982 Jul;151(1):485–486. doi: 10.1128/jb.151.1.485-486.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D., Schuldiner S., Padan E. Proton electrochemical gradient in Escherichia coli cells and its relation to active transport of lactose. Biochemistry. 1979 Feb 20;18(4):669–673. doi: 10.1021/bi00571a018. [DOI] [PubMed] [Google Scholar]


