Abstract
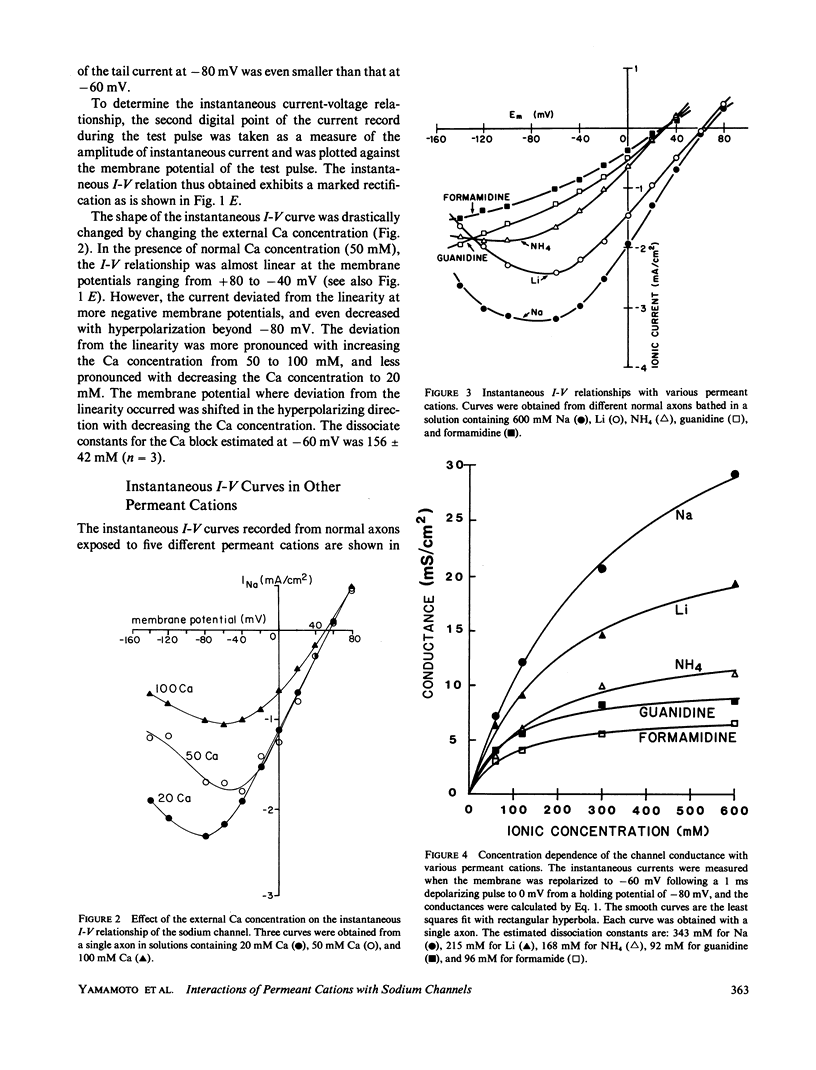
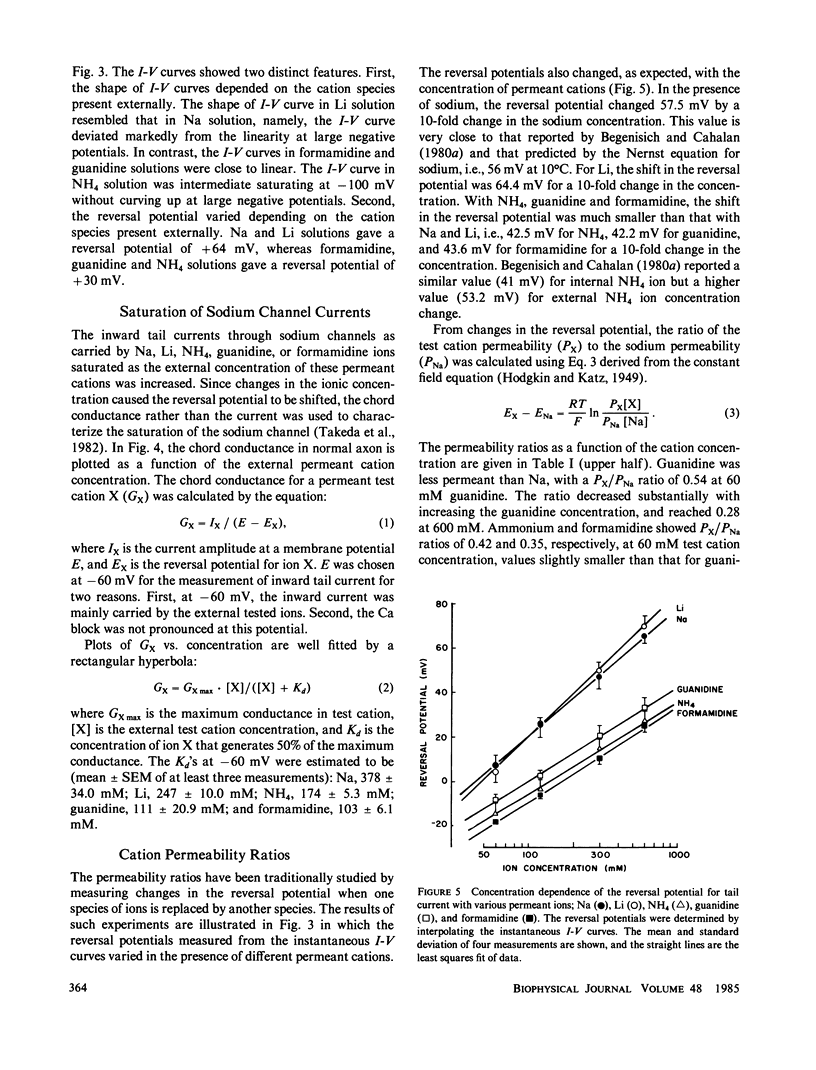
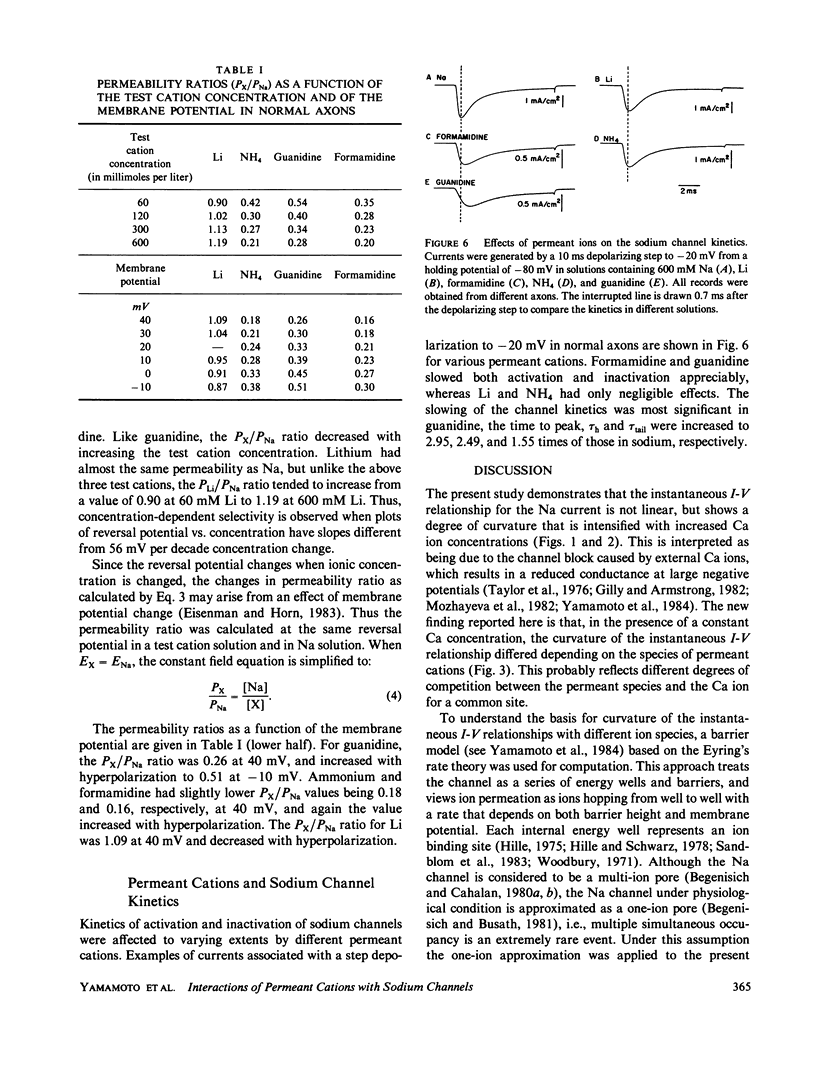
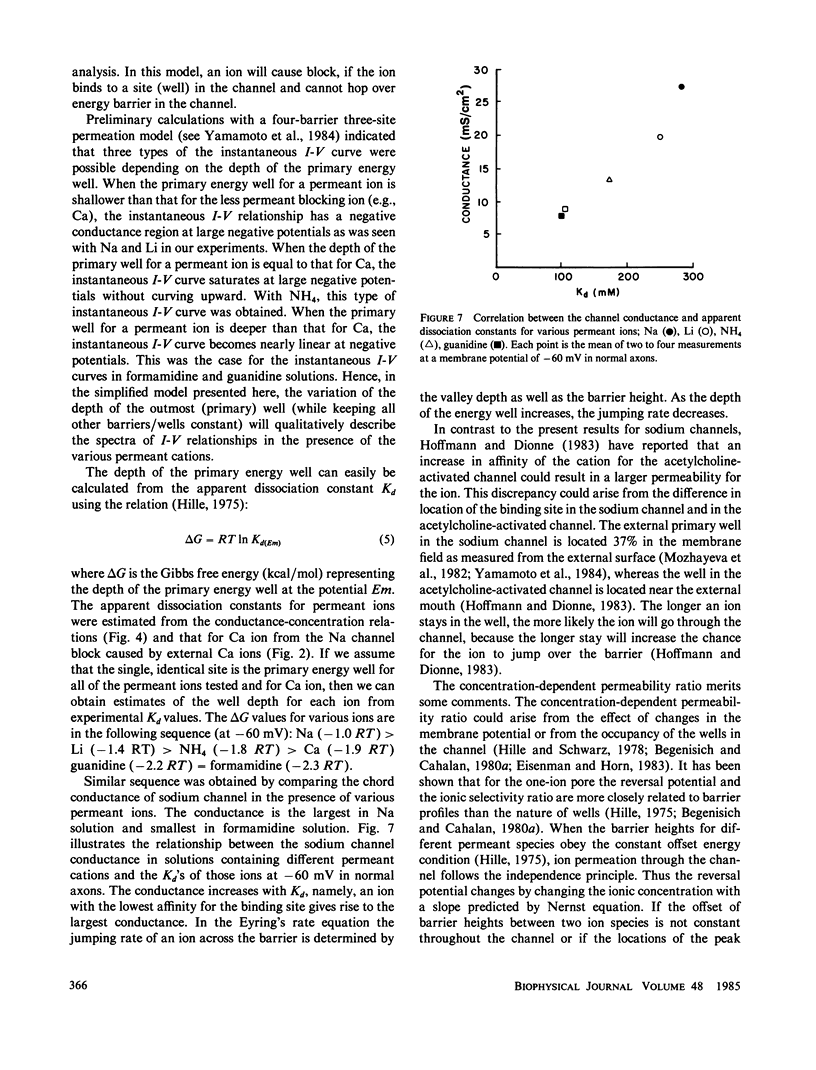
To determine how the permeant cations interact with the sodium channel, the instantaneous current-voltage (I-V) relationship, conductance-ion concentration relationship, and cation selectivity of sodium channels were studied with internally perfused, voltage clamped squid giant axons in the presence of different permeant cations in the external solution. In Na-containing media, the instantaneous I-V curve was almost linear between +60 and -20 mV, but deviated from the linearity in the direction to decrease the current at more negative potentials. The linearity of instantaneous I-V curve extended to more negative potentials with lowering the external Ca concentration. The I-V curve in Li solution was almost the same as that in Na solution. The linearity of the I-V curve improved in NH4 solution exhibiting only saturation at -100 mV with no sign of further decrease in current at more negative potentials. Guanidine and formamidine further linearized the instantaneous I-V curve. The conductance of the sodium channels as measured from the tail current saturated at high concentrations of permeant cations. The apparent dissociation constants determined from the conductance-ion concentration curve at -60 mV were as follows: Na, 378 mM; Li, 247 mM; NH4, 174 mM; guanidine, 111 mM; formamidine, 103 mM. The ratio of the test cation permeability to the sodium permeability as measured from the reversal potentials of tail currents varied with the test cation concentration and/or the membrane potential. These observations are incompatible with the independence principle, and can be explained on the basis of the Eyring's rate theory. It is suggested that the slope of the instantaneous I-V curve is determined by the relative affinity of permeant cations and blocking ions (Ca) for the binding site in the sodium channel. The ionic selectivity of the channel depends on the energy barrier profile of the channel.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Nonner W., Dwyer T. M., Hille B. Block of endplate channels by permeant cations in frog skeletal muscle. J Gen Physiol. 1981 Dec;78(6):593–615. doi: 10.1085/jgp.78.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Stanfield P. R. Calcium and potassium currents in muscle fibres of an insect (Carausius morosus). J Physiol. 1982 Feb;323:93–115. doi: 10.1113/jphysiol.1982.sp014063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the protoplasm of a giant nerve fibre with artificial solutions. Nature. 1961 Jun 3;190:885–887. doi: 10.1038/190885a0. [DOI] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. I: Reversal potential experiments. J Physiol. 1980 Oct;307:217–242. doi: 10.1113/jphysiol.1980.sp013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. B., Cahalan M. D. Sodium channel permeation in squid axons. II: Non-independence and current-voltage relations. J Physiol. 1980 Oct;307:243–257. doi: 10.1113/jphysiol.1980.sp013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., Busath D. Sodium flux ratio in voltage-clamped squid giant axons. J Gen Physiol. 1981 May;77(5):489–502. doi: 10.1085/jgp.77.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Lecar H. Ammonium ion currents in the squid giant axon. J Gen Physiol. 1969 Mar;53(3):342–361. doi: 10.1085/jgp.53.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M., Begenisich T. Sodium channel selectivity. Dependence on internal permeant ion concentration. J Gen Physiol. 1976 Aug;68(2):111–125. doi: 10.1085/jgp.68.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Voltage clamp experiments on internally perfused giant axons. J Physiol. 1965 Oct;180(4):788–820. doi: 10.1113/jphysiol.1965.sp007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Gilly W. F., Armstrong C. M. Slowing of sodium channel opening kinetics in squid axon by extracellular zinc. J Gen Physiol. 1982 Jun;79(6):935–964. doi: 10.1085/jgp.79.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473–496. doi: 10.1113/jphysiol.1952.sp004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity, saturation, and block in sodium channels. A four-barrier model. J Gen Physiol. 1975 Nov;66(5):535–560. doi: 10.1085/jgp.66.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H. M., Dionne V. E. Temperature dependence of ion permeation at the endplate channel. J Gen Physiol. 1983 May;81(5):687–703. doi: 10.1085/jgp.81.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Brodwick M. S. Acetylcholine-induced current in perfused rat myoballs. J Gen Physiol. 1980 Mar;75(3):297–321. doi: 10.1085/jgp.75.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R., Patlak J. Single channel currents from excised patches of muscle membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6930–6934. doi: 10.1073/pnas.77.11.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. A. Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol. 1979 Jan;286:417–445. doi: 10.1113/jphysiol.1979.sp012629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Anderson N. C. Mechanism of excitation block by the insecticide allethrin applied externally and internally to squid giant axons. Toxicol Appl Pharmacol. 1967 May;10(3):529–547. doi: 10.1016/0041-008x(67)90092-0. [DOI] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y., Kung C. Are ions involved in the gating of calcium channels? Science. 1982 Oct 8;218(4568):153–156. doi: 10.1126/science.6289432. [DOI] [PubMed] [Google Scholar]
- Sandblom J., Eisenman G., Hägglund J. Multioccupancy models for single filing ionic channels: theoretical behavior of a four-site channel with three barriers separating the sites. J Membr Biol. 1983;71(1-2):61–78. doi: 10.1007/BF01870675. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Bezanilla F., Taylor R. E. Sodium channel activation in the squid giant axon. Steady state properties. J Gen Physiol. 1985 Jan;85(1):65–82. doi: 10.1085/jgp.85.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Takeda K., Barry P. H., Gage P. W. Effects of extracellular sodium concentration on null potential, conductance and open time of endplate channels. Proc R Soc Lond B Biol Sci. 1982 Sep 22;216(1203):225–251. doi: 10.1098/rspb.1982.0072. [DOI] [PubMed] [Google Scholar]
- Van Helden D., Hamill O. P., Gage P. W. Permeant cations alter endplate channel characteristics. Nature. 1977 Oct 20;269(5630):711–713. doi: 10.1038/269711a0. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Yeh J. Z., Narahashi T. Voltage-dependent calcium block of normal and tetramethrin-modified single sodium channels. Biophys J. 1984 Jan;45(1):337–344. doi: 10.1016/S0006-3495(84)84159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


