Abstract
When spatial gradients of intracellular free [Ca2+] are present, intracellular calcium indicators that have a nonlinear response to [Ca2+] may yield an estimate of [Ca2+] that differs from the spatial average [Ca2+]. We present two rules that provide (a) general criteria to distinguish those classes of indicators that will yield an overestimate of spatial average [Ca2+] from those that will yield an underestimate, and (b) limits on the extent to which spatial average [Ca2+] might be over- or underestimated. These rules are used to interpret quantitatively the aequorin luminescence signals obtained from cardiac ventricular myocardium.
Full text
PDF
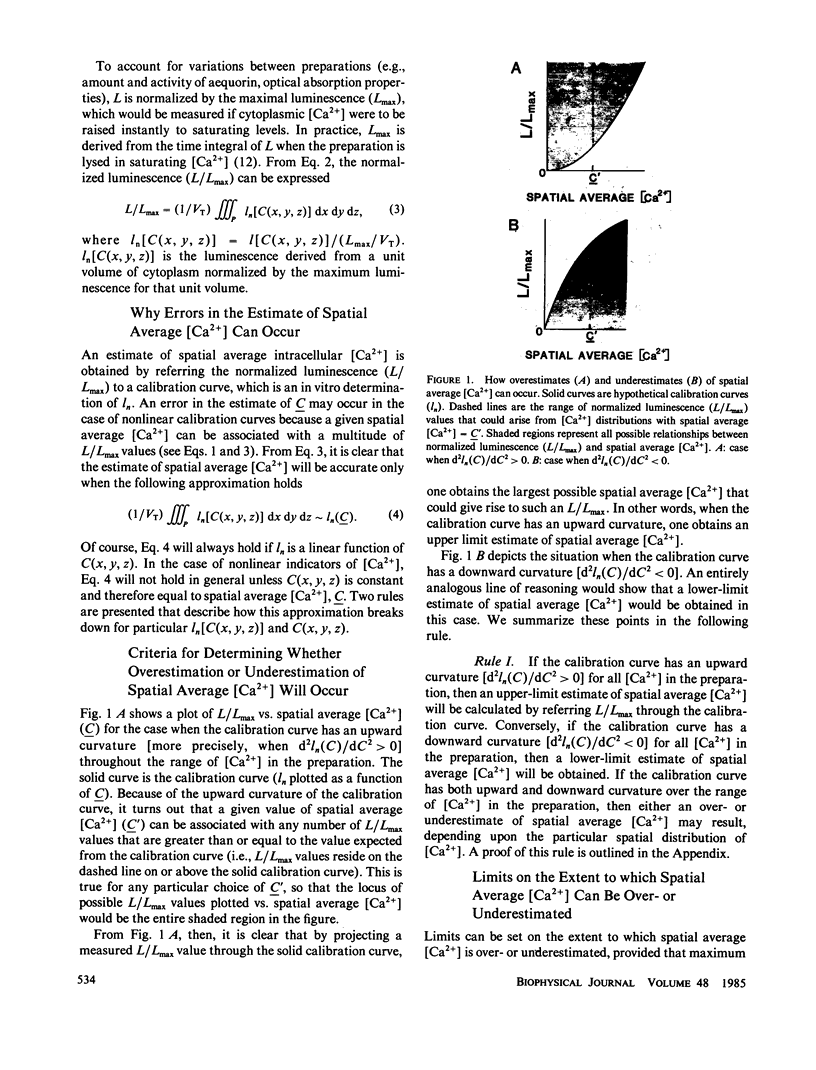
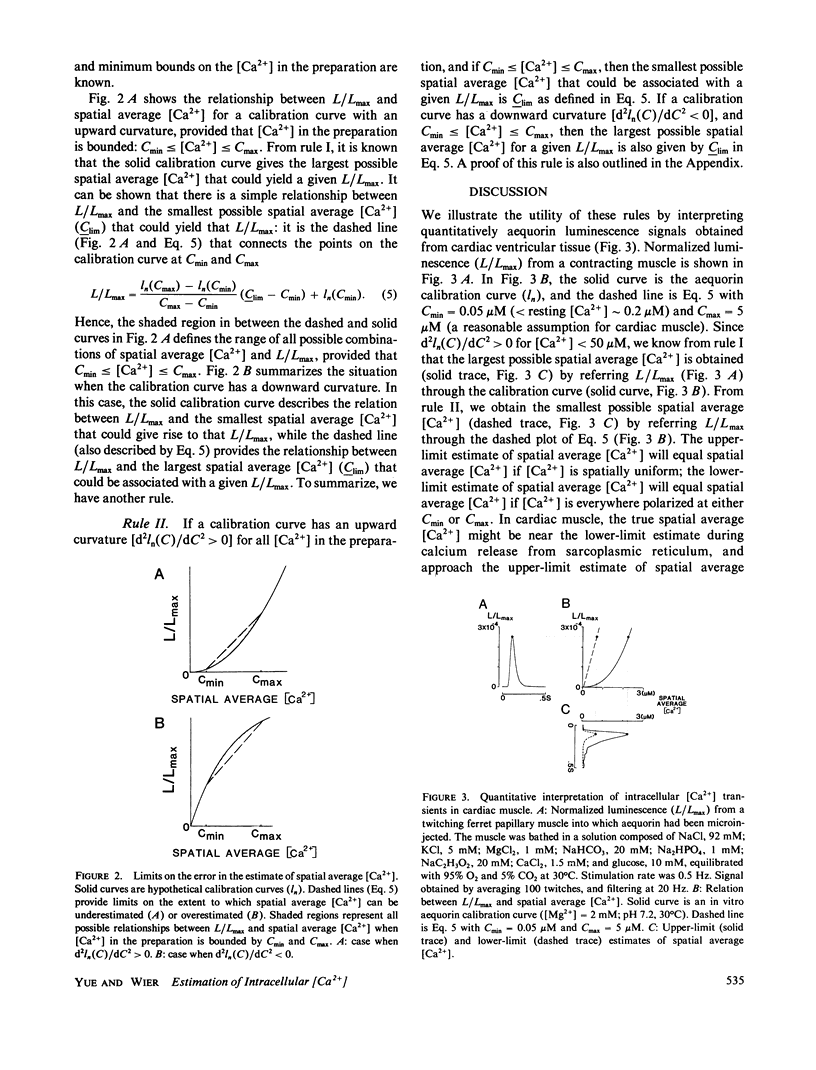


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Hodgkin A. L., Ridgway E. B. Depolarization and calcium entry in squid giant axons. J Physiol. 1971 Nov;218(3):709–755. doi: 10.1113/jphysiol.1971.sp009641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkey J. C., Jaffe L. F., Ridgway E. B., Reynolds G. T. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978 Feb;76(2):448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H. The informational role of calcium in the cytosol. Adv Cyclic Nucleotide Res. 1979;11:1–26. [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M. F. Time course of calcium release and removal in skeletal muscle fibers. Biophys J. 1984 Mar;45(3):637–641. doi: 10.1016/S0006-3495(84)84203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Calcium ion distribution in cytoplasm visualised by aequorin: diffusion in cytosol restricted by energized sequestering. Science. 1975 Dec 19;190(4220):1204–1206. doi: 10.1126/science.1198106. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Banerjee S. P. Measurement of cytosolic free calcium concentration in isolated rat ventricular myocytes with quin 2. Circ Res. 1984 Dec;55(6):830–834. doi: 10.1161/01.res.55.6.830. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Zucker R. S. Aequorin response facilitation and intracellular calcium accumulation in molluscan neurones. J Physiol. 1980 Mar;300:167–196. doi: 10.1113/jphysiol.1980.sp013157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Blinks J. R., Reynolds G. Contractile basis of ameboid movement. VII. Aequorin luminescence during ameboid movement, endocytosis, and capping. J Cell Biol. 1980 Aug;86(2):599–607. doi: 10.1083/jcb.86.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]


