Abstract
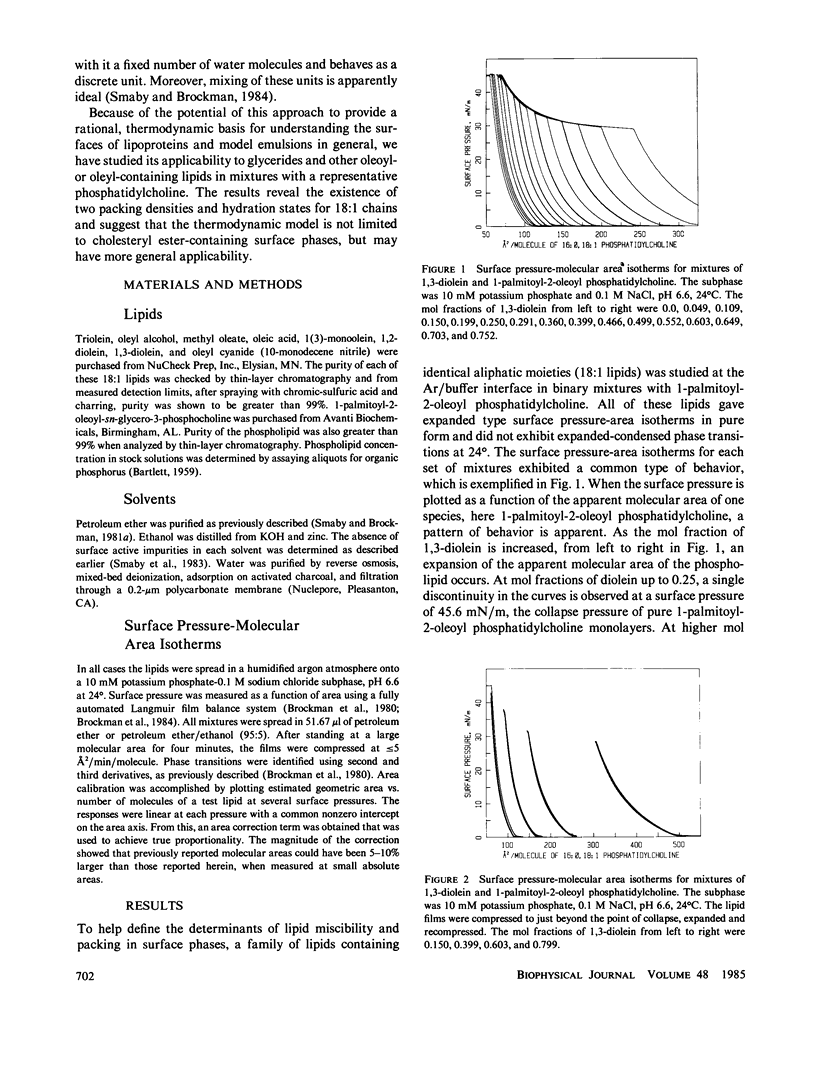
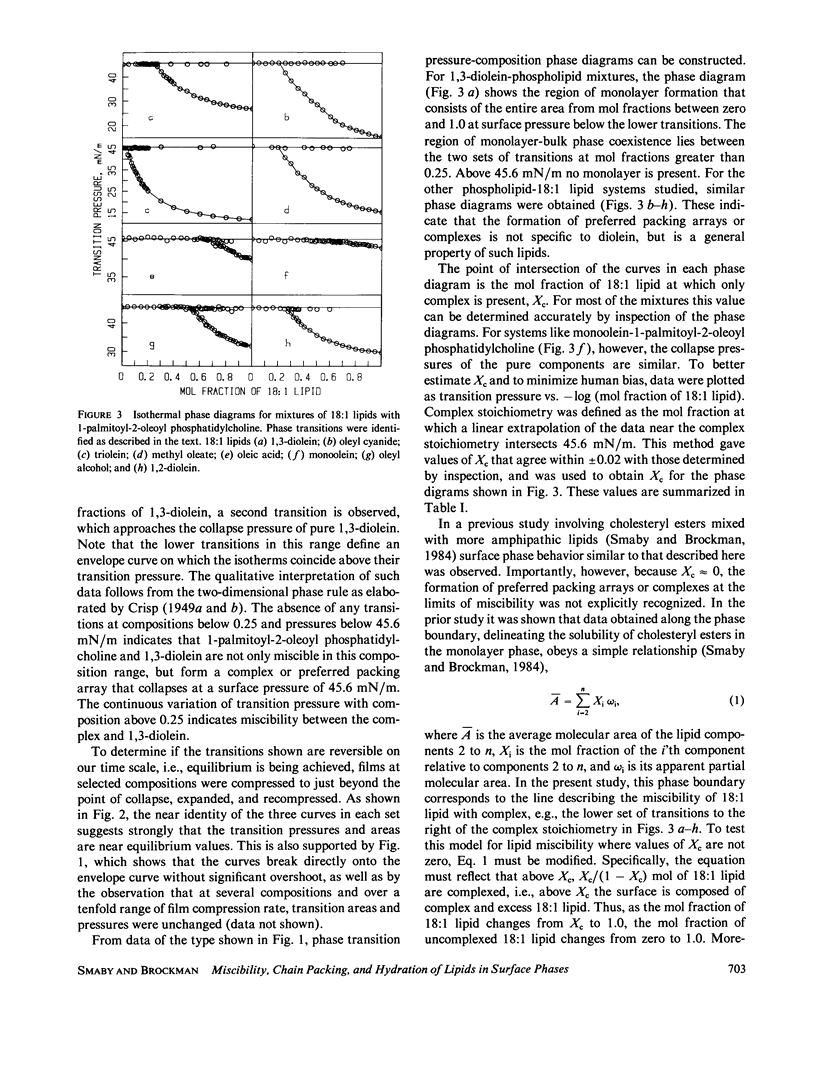
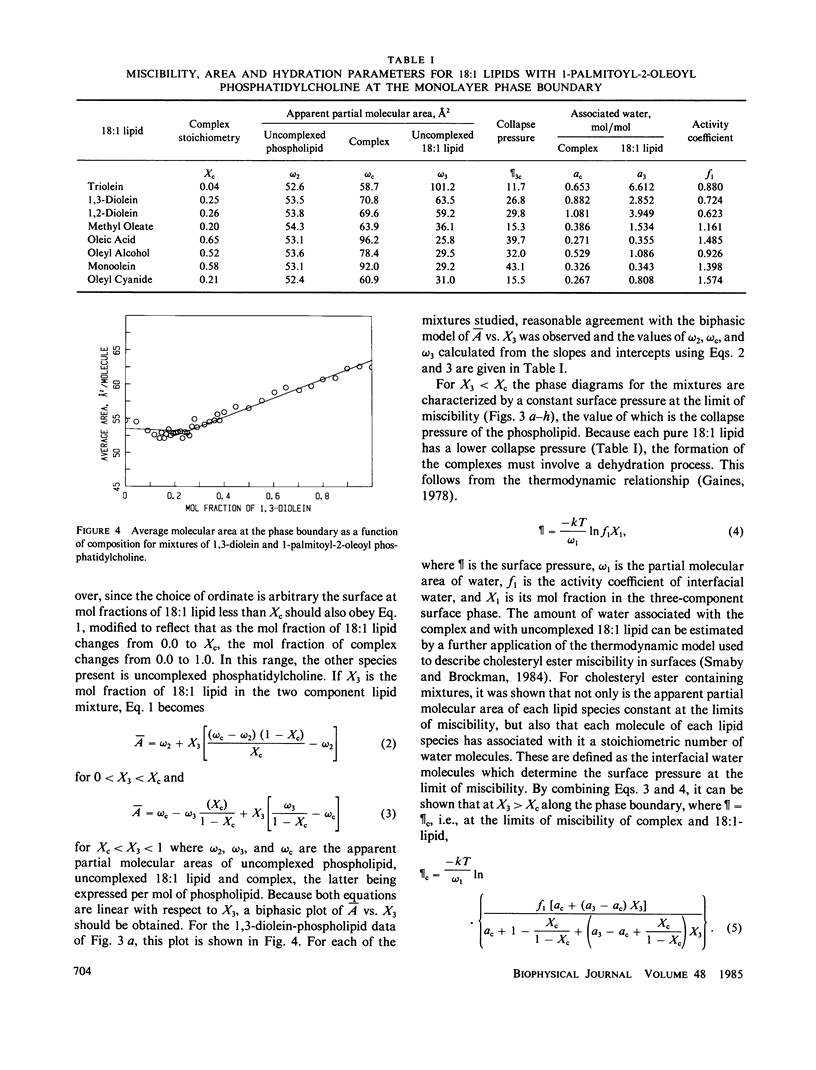
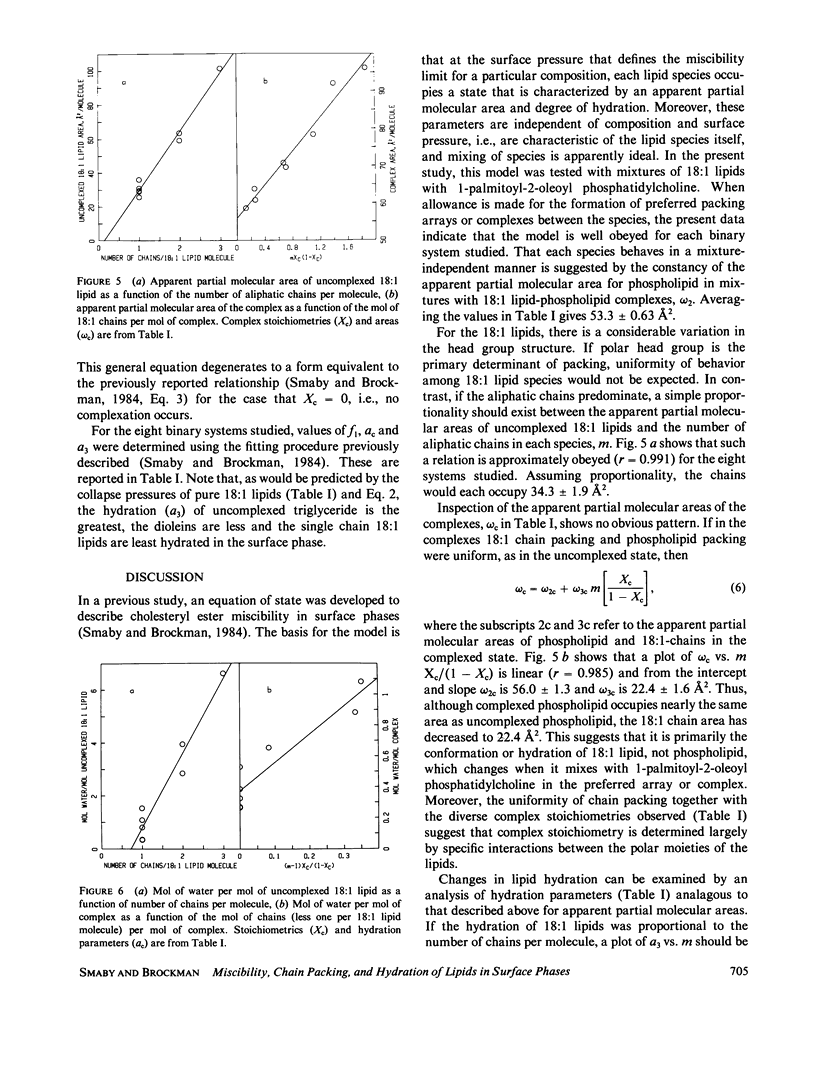
The miscibility of 1-palmitoyl-2-oleoyl phosphatidylcholine with triolein, 1,2-diolein, 1,3-diolein, 1(3)-monoolein, oleyl alcohol, methyl oleate, oleic acid, and oleyl cyanide (18:1 lipids) was studied at the argon-water interface. The isothermal phase diagrams for the mixtures at 24 degrees were characterized by two compositional regions. At the limit of miscibility with lower mol fractions of 18:1 lipid, the surface pressure was composition-independent, but above a mixture-specific stoichiometry, surface pressure at the limit of miscibility was composition-dependent. From the two-dimensional phase rule, it was determined that at low mol fractions of 18:1 lipids, the surface consisted of phospholipid and a preferred packing array or complex of phospholipid and 18:1 lipid, whereas, above the stoichiometry of the complex, the surface phase consisted of complex and excess 18:1 lipids. In both regions of the phase diagram, mixing along the phase boundary was apparently ideal allowing application of an equation of state described earlier (J. M. Smaby and H. L. Brockman, 1984, Biochemistry, 23:3312-3316). From such analysis, apparent partial molecular areas and hydrations for phospholipid, complex, and 18:1 lipid were obtained. Comparison of these calculated parameters for the complexed and uncomplexed states shows that the aliphatic moieties behave independently of polar head group. The transition of each 18:1 chain to the complexed state involves the loss of about one interfacial water molecule and its corresponding area. For 18:1 lipids with more than one chain another two water molecules per additional chain are present in both states but contribute little to molecular area. In contrast to 18:1 lipids, the phospholipid area and hydration change little upon complexation. The uniformity of chain packing and hydration behavior among 18:1 lipid species contrasts with complex stoichiometries that vary from 0.04 to 0.65. This suggests that the stoichiometry of the preferred packing array is determined by interactions involving the more polar moieties of the 18:1 lipids and the phospholipid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Demel R. A., Shirai K., Jackson R. L. Lipoprotein lipase-catalyzed hydrolysis of tri[14C]oleoylglycerol in a phospholipid interface. A monolayer study. Biochim Biophys Acta. 1982 Dec 13;713(3):629–637. doi: 10.1016/0005-2760(82)90323-x. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Miller K. W., Small D. M. Solubilization of triolein and cholesteryl oleate in egg phosphatidylcholine vesicles. J Biol Chem. 1983 Nov 10;258(21):12821–12826. [PubMed] [Google Scholar]
- Miller K. W., Small D. M. Surface-to-core and interparticle equilibrium distributions of triglyceride-rich lipoprotein lipids. J Biol Chem. 1983 Nov 25;258(22):13772–13784. [PubMed] [Google Scholar]
- Miller K. W., Small D. M. Triolein-cholesteryl oleate-cholesterol-lecithin emulsions: structural models of triglyceride-rich lipoproteins. Biochemistry. 1983 Jan 18;22(2):443–451. doi: 10.1021/bi00271a030. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Novel surface phase containing cholesteryl esters. 1. Structural characteristics determined from surface pressure--area measurements. Biochemistry. 1981 Feb 17;20(4):718–723. doi: 10.1021/bi00507a008. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Novel surface phase containing cholesteryl esters. 2. Nonequivalence of cholesteryl arachidonate and those with 18-carbon, cis-unsaturated acyl groups. Biochemistry. 1981 Feb 17;20(4):724–730. doi: 10.1021/bi00507a009. [DOI] [PubMed] [Google Scholar]
- Smaby J. M., Brockman H. L. Thermodynamic equation of state for cholesteryl esters in surface phases. Biochemistry. 1984 Jul 3;23(14):3312–3317. doi: 10.1021/bi00309a030. [DOI] [PubMed] [Google Scholar]
- Small D. M. Lateral chain packing in lipids and membranes. J Lipid Res. 1984 Dec 15;25(13):1490–1500. [PubMed] [Google Scholar]
- Small D. M., Shipley G. G. Physical-chemical basis of lipid deposition in atherosclerosis. Science. 1974 Jul 19;185(4147):222–229. doi: 10.1126/science.185.4147.222. [DOI] [PubMed] [Google Scholar]


