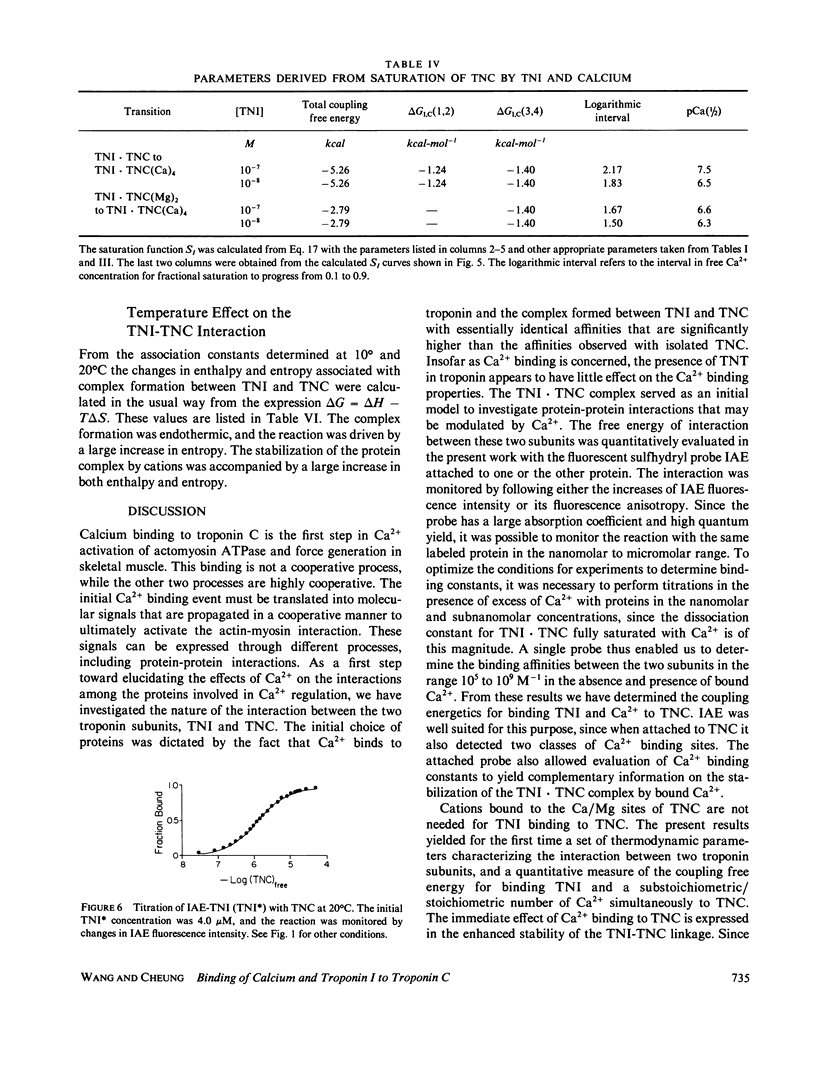
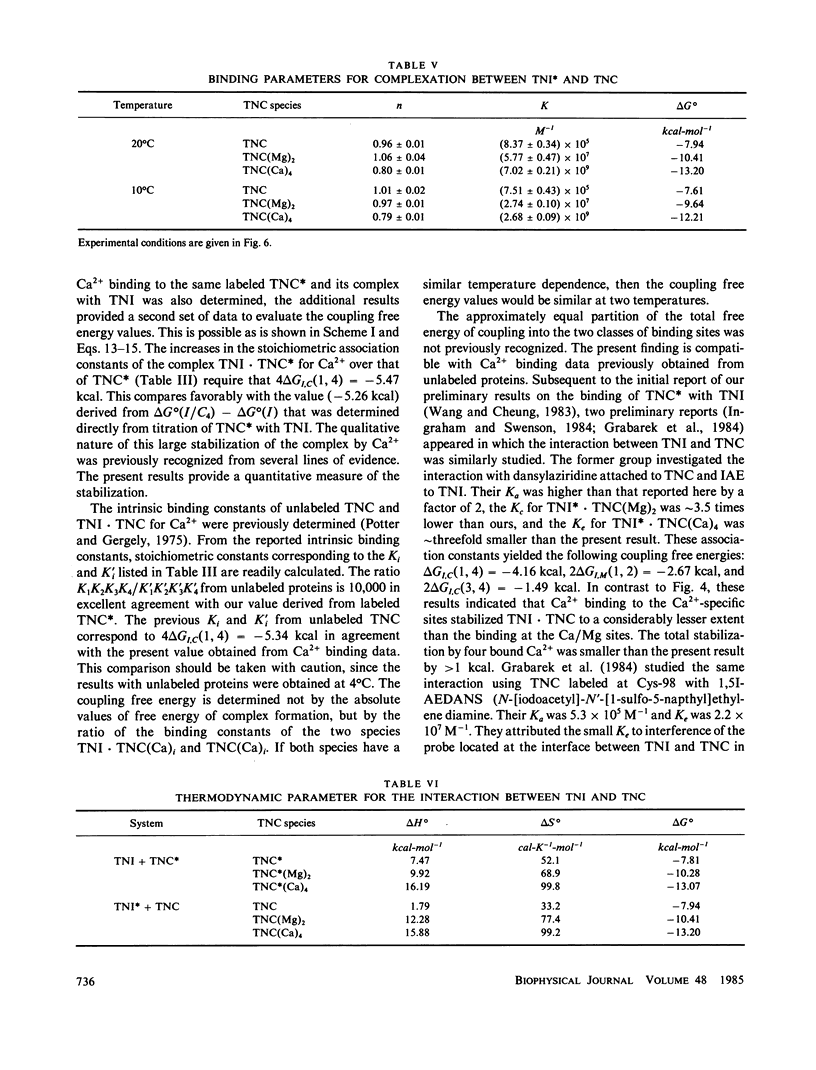
Abstract
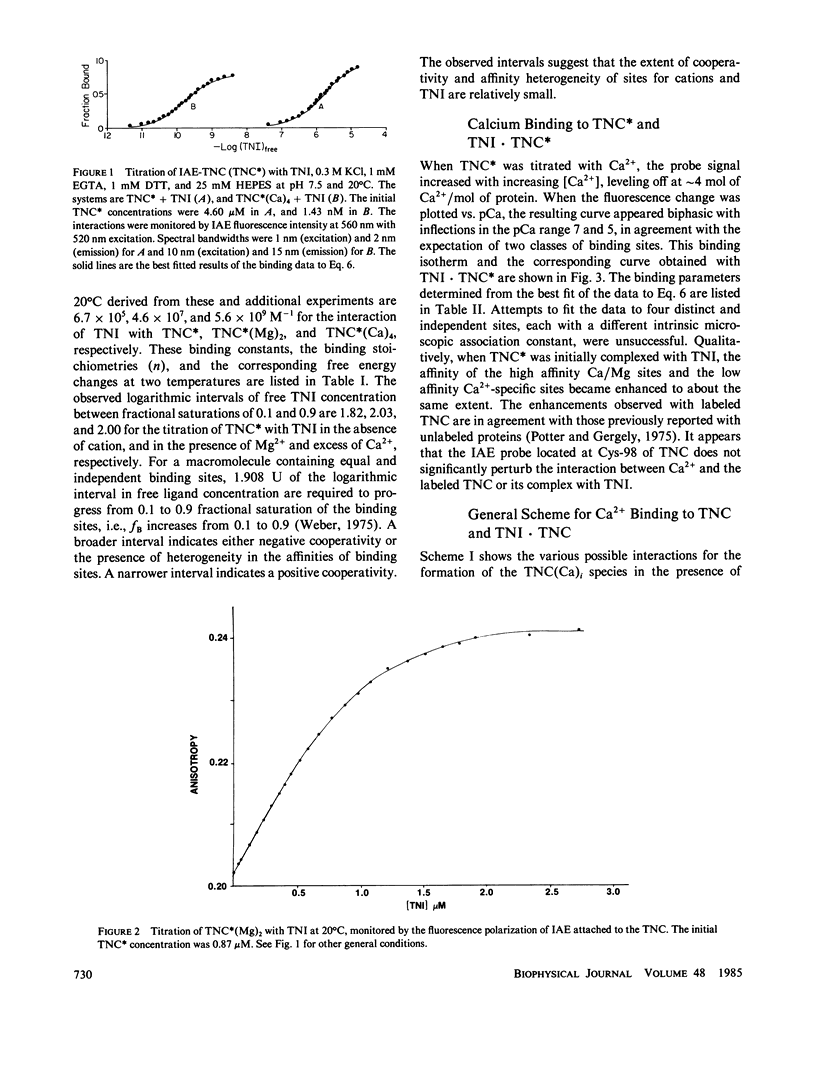
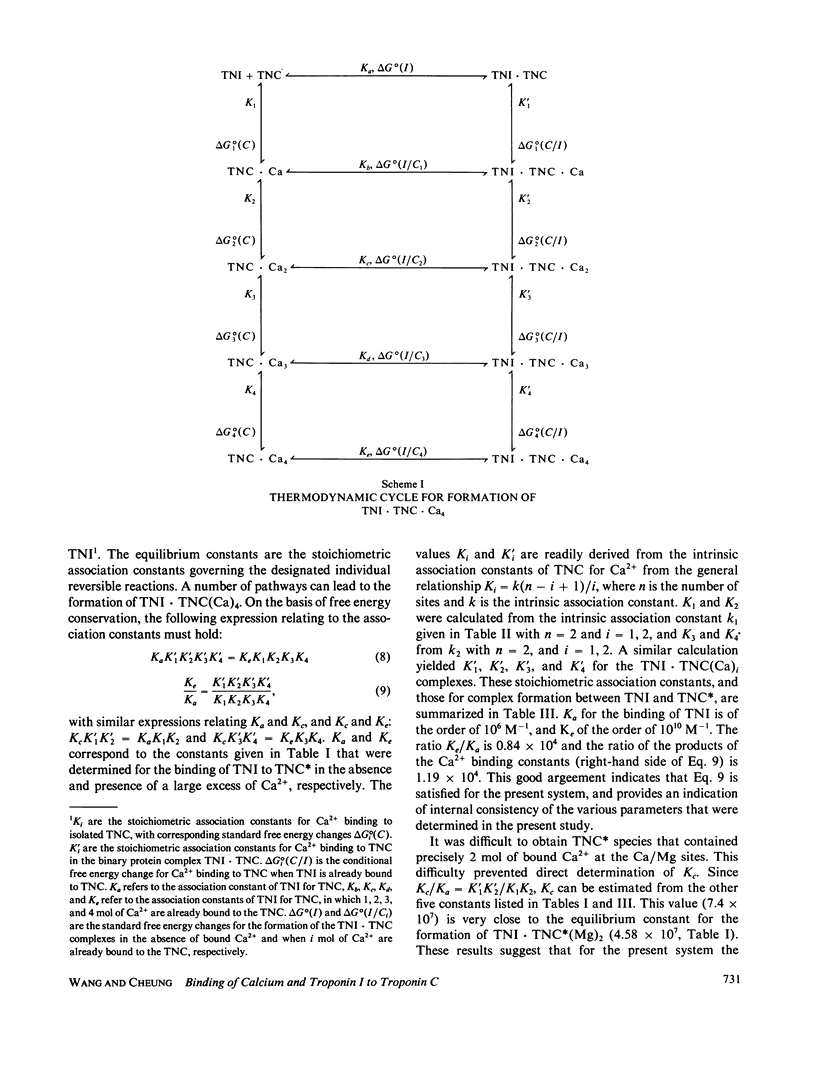
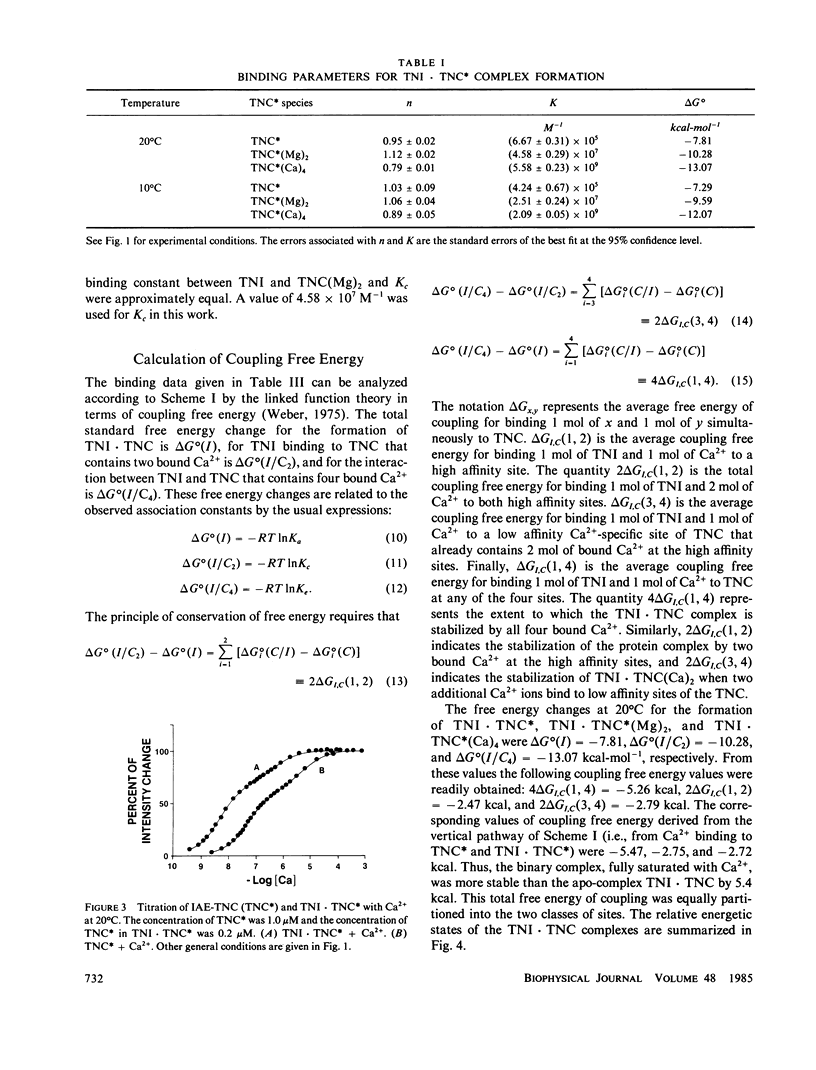
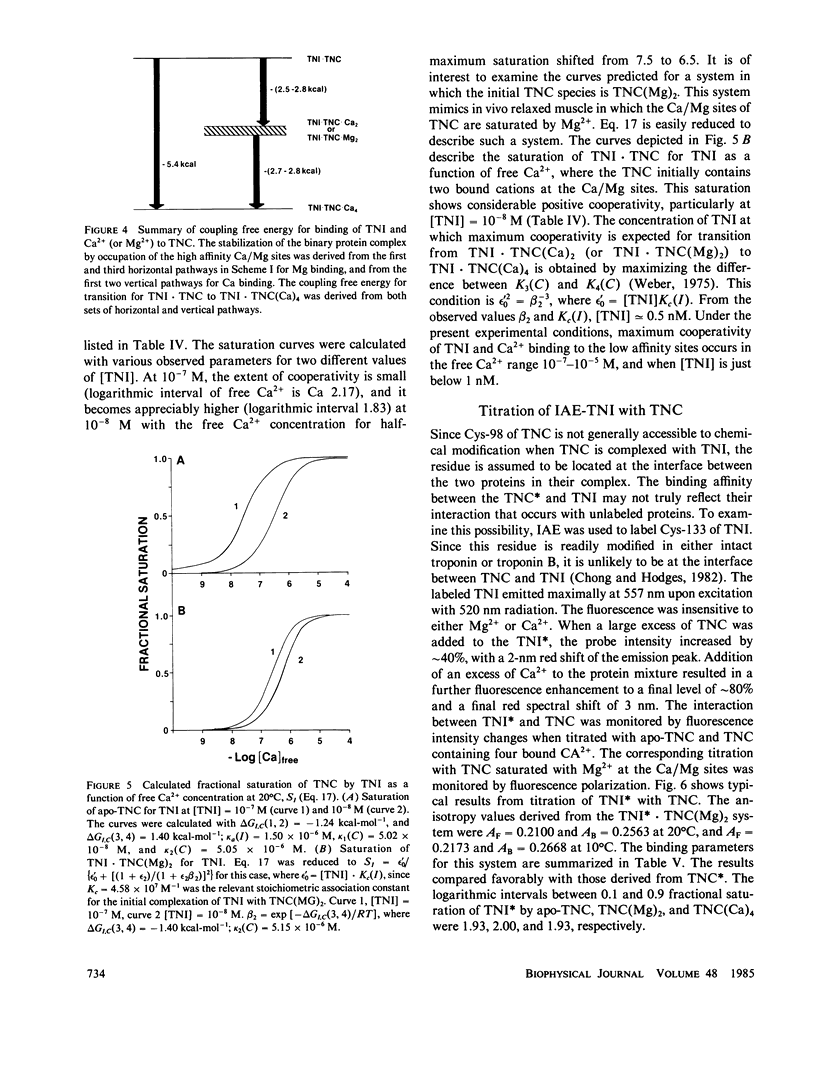
We determined the free energy of interaction between rabbit skeletal troponin I (TNI) and troponin C (TNC) at 10 degrees and 20 degrees C with fluorescently labeled proteins. The sulfhydryl probe 5-iodoacetamidoeosin (IAE) was attached to cysteine (Cys)-98 of TNC and to Cys-133 of TNI, and each of the labeled proteins was titrated with the other unlabeled protein. The association constant for formation of the complex between labeled TNC (TNC*) and TNI was 6.67 X 10(5) M-1 in 0.3 M KCl, and pH 7.5 at 20 degrees C. In the presence of bound Mg2+, the binding constant increased to 4.58 X 10(7) M-1 and in the presence of excess of Ca2+, the association constant was 5.58 X 10(9) M-1. Very similar association constants were obtained when labeled TNI was titrated with unlabeled TNC. The energetics of Ca2+ binding to TNC* and the complex TNI X TNC* were also determined at 20 degrees C. The two sets of results were used to separately determine the coupling free energy for binding TNI and Mg2+, or Ca2+ to TNC. The results yielded a total coupling free energy of -5.4 kcal. This free energy appeared evenly partitioned into the two species: TNI X TNC(Mg)2 or TNI X TNC(Ca)2, and TNI X TNC(Ca)4. The first two species were each stabilized by -2.6 kcal, with respect to the Ca2+ free TNI X TNC complex, and TNI X TNC(Ca)4 was stabilized by -2.8 kcal, respect to TNI X TNC(Ca)2 or TNI X TNC(Mg)2. The coupling free energy was shown to produce cooperatively complexes formed between TNI and TNC in which the high affinity sites were initially saturated as a function of free Ca2+ to yield TNI X TNC(Ca)4. This saturation occurred in the free Ca2+ concentration range 10(-7) to 10(-5) M. The cooperative strengthening of the linkage between TNI and TNC induced by Ca2+ binding to the Ca2+-specific sites of TNC may have a direct relationship to activation of actomyosin ATPase. The nature of the forces involved in the Ca2+-induced strengthening of the complex is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brzeska H., Szynkiewicz J., Drabikowski W. Localization of hydrophobic sites in calmodulin and skeletal muscle troponin C studied using tryptic fragments: a simple method of their preparation. Biochem Biophys Res Commun. 1983 Aug 30;115(1):87–93. doi: 10.1016/0006-291x(83)90972-5. [DOI] [PubMed] [Google Scholar]
- Cachia P. J., Sykes B. D., Hodges R. S. Calcium-dependent inhibitory region of troponin: a proton nuclear magnetic resonance study on the interaction between troponin C and the synthetic peptide N alpha-acetyl[FPhe106]TnI-(104-115) amide. Biochemistry. 1983 Aug 16;22(17):4145–4152. doi: 10.1021/bi00286a024. [DOI] [PubMed] [Google Scholar]
- Cheung H. C., Wang C. K., Garland F. Fluorescence energy transfer studies of skeletal troponin C proximity between methionine-25 and cysteine-98. Biochemistry. 1982 Oct 12;21(21):5135–5142. doi: 10.1021/bi00264a005. [DOI] [PubMed] [Google Scholar]
- Chong P. C., Hodges R. S. Proximity of sulfhydryl groups to the sites of interaction between components of the troponin complex from rabbit skeletal muscle. J Biol Chem. 1982 Mar 10;257(5):2549–2555. [PubMed] [Google Scholar]
- Chothia C., Janin J. Principles of protein-protein recognition. Nature. 1975 Aug 28;256(5520):705–708. doi: 10.1038/256705a0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. A colorimetric method for determining low concentrations of mercaptans. Arch Biochem Biophys. 1958 Apr;74(2):443–450. doi: 10.1016/0003-9861(58)90014-6. [DOI] [PubMed] [Google Scholar]
- Gariépy J., Sykes B. D., Reid R. E., Hodges R. S. Proton nuclear magnetic resonance investigation of synthetic calcium-binding peptides. Biochemistry. 1982 Mar 30;21(7):1506–1512. doi: 10.1021/bi00536a007. [DOI] [PubMed] [Google Scholar]
- Grabarek Z., Drabikowski W., Leavis P. C., Rosenfeld S. S., Gergely J. Proteolytic fragments of troponin C. Interactions with the other troponin subunits and biological activity. J Biol Chem. 1981 Dec 25;256(24):13121–13127. [PubMed] [Google Scholar]
- Grabarek Z., Grabarek J., Leavis P. C., Gergely J. Cooperative binding to the Ca2+-specific sites of troponin C in regulated actin and actomyosin. J Biol Chem. 1983 Dec 10;258(23):14098–14102. [PubMed] [Google Scholar]
- Grand R. J., Levine B. A., Perry S. V. Proton-magnetic-resonance studies on the interaction of rabbit skeletal-muscle troponin I with troponin C and actin. Biochem J. 1982 Apr 1;203(1):61–68. doi: 10.1042/bj2030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iio T., Kondo H. Fluorescence titration and fluorescence stopped-flow studies on skeletal troponin C labeled with fluorescent maleimide reagent or dansylaziridine. J Biochem. 1981 Jul;90(1):163–175. doi: 10.1093/oxfordjournals.jbchem.a133446. [DOI] [PubMed] [Google Scholar]
- Janin J., Chothia C. Role of hydrophobicity in the binding of coenzymes. Appendix. Translational and rotational contribution to the free energy of dissociation. Biochemistry. 1978 Jul 25;17(15):2943–2948. doi: 10.1021/bi00608a001. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Charlton S. C., Potter J. D. A fluorescence stopped flow analysis of Ca2+ exchange with troponin C. J Biol Chem. 1979 May 10;254(9):3497–3502. [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Leavis P. C., Rosenfeld S. S., Gergely J., Grabarek Z., Drabikowski W. Proteolytic fragments of troponin C. Localization of high and low affinity Ca2+ binding sites and interactions with troponin I and troponin T. J Biol Chem. 1978 Aug 10;253(15):5452–5459. [PubMed] [Google Scholar]
- Levine B. A., Coffman D. M., Thornton J. M. Calcium binding by troponin-C. A proton magnetic resonance study. J Mol Biol. 1977 Oct 5;115(4):743–760. doi: 10.1016/0022-2836(77)90113-9. [DOI] [PubMed] [Google Scholar]
- Murray A. C., Kay C. M. Hydrodynamic and optical properties of troponin A. Demonstration of a conformational change upon binding calcium ion. Biochemistry. 1972 Jul 4;11(14):2622–2627. doi: 10.1021/bi00764a012. [DOI] [PubMed] [Google Scholar]
- Nagy B., Potter J. D., Gergely J. Calcium-induced conformational changes in a cyanogen bromide fragment of troponin C that contains one of the binding sites. J Biol Chem. 1978 Sep 10;253(17):5971–5974. [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V. The regulation of contractile activity in muscle. Biochem Soc Trans. 1979 Aug;7(4):593–617. doi: 10.1042/bst0070593. [DOI] [PubMed] [Google Scholar]
- Potter J. D., Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase. J Biol Chem. 1975 Jun 25;250(12):4628–4633. [PubMed] [Google Scholar]
- Reid R. E., Gariépy J., Saund A. K., Hodges R. S. Calcium-induced protein folding. Structure-affinity relationships in synthetic analogs of the helix-loop-helix calcium binding unit. J Biol Chem. 1981 Mar 25;256(6):2742–2751. [PubMed] [Google Scholar]
- Talbot J. A., Hodges R. S. Synthetic studies on the inhibitory region of rabbit skeletal troponin I. Relationship of amino acid sequence to biological activity. J Biol Chem. 1981 Mar 25;256(6):2798–2802. [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M. The preparation and properties of the components of troponin B. Biochim Biophys Acta. 1974 Aug 8;359(2):379–388. doi: 10.1016/0005-2795(74)90238-4. [DOI] [PubMed] [Google Scholar]


